Pool pH and Total Alkalinity are crucial for protecting pool surfaces and providing sanitary water conditions. However, they can be challenging to manage unless you understand what they are and their role in overall pool balance. In this post, we’ll go over exactly what pH and Total Alkalinity are, why they’re important for the health of your pool, the impact of high or low pH/Total Alkalinity, and how to address tough issues that might arise from an imbalance. Class is now in session – let’s dive in!
What is pH?
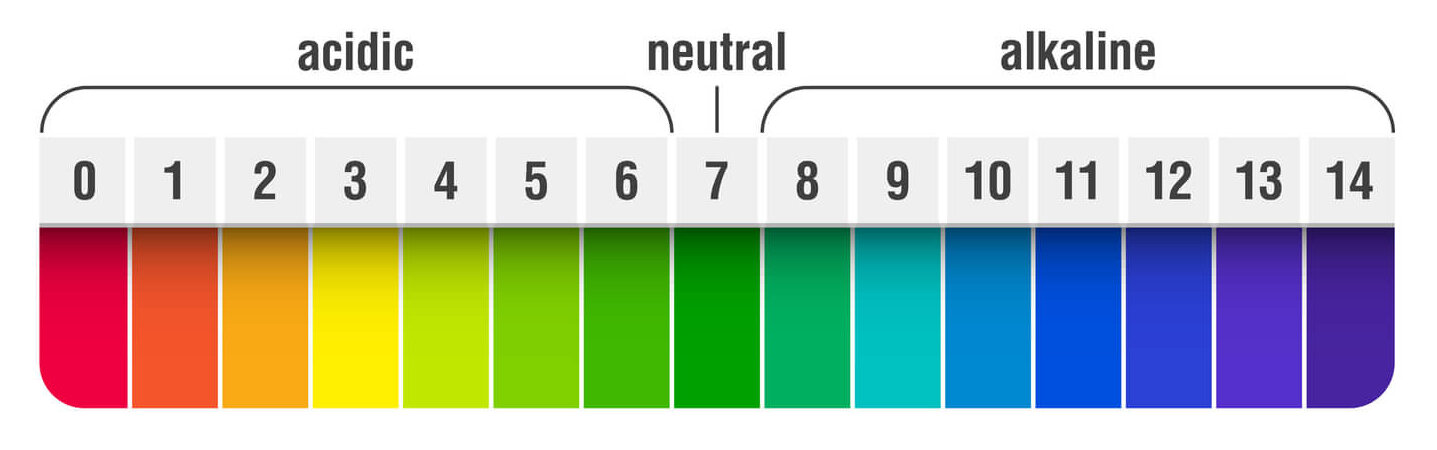
The pH balance in your swimming pool is arguably the single most important factor in maintaining clear, comfortable water. It is a measurement of how acidic or basic the water is, and is based on a scale from 0-14, with a pH reading of 7 being neutral. Anything below 7 is considered acidic, while anything above 7 is considered basic or alkaline. For swimming pools, the ideal pH range is 7.4-7.6 (7.2-7.8 acceptable).
A proper pH reading will keep swimmers’ skin and eyes comfortable, and protects pool surfaces against corrosive damage, stains and scale buildup. Balanced pH allows chlorine to do its job most effectively, keeping your pool clean, clear, and algae-free. As a general rule of thumb, pH can be adjusted with the following:
- 1 oz. of pH Increaser (soda ash or sodium carbonate) added to 1,000 gallons will raise pH by 0.1.
- 1 oz. of pH Reducer (sodium bisulfate) added to 1,000 gallons will lower pH by 0.1.
When balancing pH, always consult the product label for guidelines and instructions. It can be easy to miss the mark when adjusting pool pH, as pH adjustment can be affected by the temperature, Total Alkalinity, and Calcium Hardness levels of your water. Try not to overdose the pool, and always test pH level a few hours after adding pH chemicals to confirm that you’ve restored proper balance.
What is Total Alkalinity?
Total Alkalinity is very closely related to pH, as it is a measurement of all alkaline substances in the water. Proper Alkalinity levels are a key factor in maintaining pH – many describe it as being a “pH buffer.” Low Total Alkalinity levels causes rapid pH fluctuations, and high levels make pH difficult to adjust. That said, it’s best to adjust one and then the other, not both at the same time. If Total Alkalinity is out of balance, address it first.
The best range for Total Alkalinity depends which sanitizer you use. If using calcium hypochlorite (cal-hypo), sodium hypochlorite, or lithium hypochlorite as your primary sanitizer, the ideal TA range is 80-100 ppm. When using dichlor, trichlor, or bromine as your primary sanitizer, the ideal range is between 100-120 ppm. For a salt water chlorine generator, most manufacturers recommend TA levels between 80-120 ppm. Maintaining the right balance of pH and Total Alkalinity is essential to keeping your pool water in good condition.

If your Total Alkalinity levels are too low (below 80 ppm) but pH levels are above 6.8, you will need to add Alkalinity Increaser (sodium bicarbonate). When both Total Alkalinity and pH are too low (TA less than 80 ppm, pH below 6.8), use pH Increaser (sodium carbonate) to bring both levels up. If your Total Alkalinity levels are too high, add pH Reducer (sodium bisulfate).
Lowering Total Alkalinity levels can be a lengthy, sometimes frustrating process, as sodium bisulfate will also have the effect of reducing your pH at the same time as it lowers your Total Alkalinity. You may need to make repeated adjustments of lowering Alkalinity and then raising pH until both come into proper range. No matter what adjustments you need to make, always retest the water with test strips or a test kit before adding more chemicals. Again, always follow dosing and application instructions from the product label as you move levels gradually into the proper range.
Why Are pH and Total Alkalinity Important for My Pool?
Unbalanced pH and Total Alkalinity can quickly throw your whole pool into a downward spiral, costing you loads of time and money on cleaning, repairs, and extra chemicals.
Low pH in Pools
- Etching and erosion of grout and plaster
- Vinyl liners become stiff and brittle
- Solar covers and pool floats are damaged
- Corrosion and oxidation of metals on ladders, lights, and heat exchangers
- Plastic fittings (returns, skimmer faceplates) become brittle
- Swimmer comfort issues, including burning eyes and dry skin
- Free Available Chlorine dissipates faster than normal
High pH in Pools
- Chlorine is around 50% less effective at pH levels over 8.2
- Scaling from calcium deposits on pool surfaces and waterline
- Algae blooms likely
- Pool water may appear cloudy and dull
Low Total Alkalinity in Pools
- Etching of pool plaster
- Vinyl liners become stiff and brittle
- Corrosion of metals in ladders, handrails, and heat exchangers
- Swimmer comfort issues, including burning eyes and dry skin
- Pool surface stains
- Rapid fluctuations in pH, known as “pH bounce”
High Total Alkalinity in Pools
- Decreased chlorine effectiveness
- Frequent problems with cloudy water
- Stain and scale formation on pool surfaces
- Difficulty adjusting pH level – pH increases despite regular addition of pH reducer
Common Problems with Pool pH and Total Alkalinity

Now that you have a basic understanding of how Total Alkalinity and pH work together, let’s take a closer look at how to resolve specific balance issues in your pool. We’ve said it before, but we’ll say it again – when making any adjustments to your water’s chemistry, ALWAYS read and follow product label instructions, and only add balancing chemicals to the pool if the pump is running. Start with an accurate pool water test to ensure you’re dosing the pool correctly. And remember: it’s far better to make small, gradual adjustments than it is to make large changes. Keep on top of pool water chemistry with regular testing 2-3 times per week.
Pool pH is Always High
The most common reason for a consistently high pH level in pools is the use of liquid chlorine or a saltwater system as the primary sanitizer. Sodium hydroxide is produced, which has a pH of around 13. New pool plaster or pebble finishes will also raise pH in pools for about a year after installation. If your pool has water features, such as a waterfall or fountain, these will also raise pH levels in your pool (more on that below).
Pool pH is Always Low
The most common cause of a consistently low pH level in pools is using chlorine tablets or stabilized forms of chlorine. These have a pH level of around 3. Acidic rainfall, heavy leaf debris, and dirt/mulch in the pool can also lower the pH level.
Pool pH is Low, Total Alkalinity is High
High Total Alkalinity over 180 ppm can cause some resistance to pH change. Adding pH Increaser can also raise Total Alkalinity, compounding the problem. Make repeated adjustments of lowering Alkalinity and then raising pH until both come into proper range, testing the water each time before adding more chemicals.
Pool pH is High, Total Alkalinity is Low
Low Total Alkalinity under 80 ppm can cause pH to be unstable and erratic. To raise it, add Alkalinity Increaser (add 1 lb. per 10,000 gals to increase by 10 ppm). Keep in mind that this will also slightly raise your pool’s pH level, but not as much as the Total Alkalinity level. Once TA is back in balance, you can adjust pH. Just like in the scenario above, you may need to make repeated alternating adjustments to get the levels just right.
Pool pH and Total Alkalinity are Both High
To lower both pH and Total Alkalinity you only need pH Reducer, otherwise known as dry acid. Muriatic acid, Acid Magic, and No Mor Acid can also be used to lower Total Alkalinity and pH levels in pools.
Pool pH and Total Alkalinity are Both Low
To raise pH and Total Alkalinity at the same time, use pH Increaser. Be sure to test for the presence of metals first, and address those issues before adding product to the pool.
Pool pH will not Increase or Decrease
If it seems like you keep adding pH Increaser or pH Reducer, but it only seems to last a day (if it even has effect at all), high Total Alkalinity levels over 180 ppm may be preventing you from making an effective adjustment. For pools with high Total Alkalinity and high pH levels, add pH Reducer or other acid to affect both.
Is it Safe to Swim in High or Low pH or Alkalinity?
Maybe yes, maybe no. Your pool water pH and Total Alkalinity can affect disinfection by chlorine. Chlorine is very slow to react at high pH levels, and can dissipate quickly at low pH levels. However, a pool could still be sanitary with good levels of Free Available Chlorine and effective filtration. However, the water may be uncomfortable for the skin and eyes, and will damage your pool over time. It’s important to address chemical imbalances as quickly as possible to keep your pool experience pleasant.
Bonus Tips
Use Aeration to Raise Pool pH

Looking to raise pH without affecting Total Alkalinity? You can sometimes raise the pH through aerating the pool water. Yep, just add air! It’s the same reason hot tubs and spas often suffer from high pH issues. Although this will raise your pH, it’s important to note that this isn’t an exact science, and results will vary by pool.
You can aerate a pool by bubbling air through the water, agitating the surface, spraying droplets through the air with a pool fountain, or even just playing and splashing around in the pool. During the hot summer months, this method also can be used to lower pool water temperature.
Getting an Accurate Water Test
One more tip! For correct pH and Total Alkalinity testing, a titration test is generally much more accurate than test strips. We like to recommend the Taylor K-2005, but if the price tag is too much, take a look at the less expensive Swimline 4-in-1 for a reliable pool pH and Alkalinity test kit.
If you have any other questions about pool pH or Total Alkalinity chemicals that we didn’t cover, let us know. You can also check out our handy dosage charts for more information about how to reach specific levels with water balancing chemicals.











Please help! The chemicals in pool are out of wack and i follow the directions from leslie Pool Supply. Readings are as follows: Free Chlorine = 10, Total Chlorine = 10,pH = 7.8, Total Alkalinity = 50, Calcium Hardness = 130, Cyanuric Acid= 90. Instructed to add 6lbs 4oz of Alkalinity Up first( waited 4 hours to test and its 180 via test strips). instructed to add 13 lbs 6oz of Hardness Plus the following day and add 14 oz of Chlor Neutralizer ( added 6.4oz of Chlor Neutralizer one day earlier but chlorine still elevated). Dont understand how chlorine increases so much concidering I turned off the chlorinator a few days earlier and did not add any chlorine. I am a new pool owner. The pool was completed at the end of August and is 8500 gallons of fresh water. I test the water a couple times a week and the ph has always been elevated. this is the first issue with chlorine being high and alkalinity being low.Don’t understand what i am doing wrong.
hi Steven, primarily the chlorine is high because the cyanuric acid is so high (90 ppm). We recommend 30-50 ppm, and at 90 ppm, the chlorine is not burning off from the sun. To lower the cyanuric acid, you would need to replace about half of the water in the pool (the solution is dilution). Replacing half the water will lower cyanuric acid by half, or to 45 ppm. The high pH readings are normal for a new (plaster) pool, and will stabilize after the first year. Try to keep pH below 7.8, and is best around 7.4. When you continually add pH decreaser, this will also lower alkalinity, so you will need to add alk increaser every month or so, to keep it above 80 ppm.
I apologize for the novice question. I have done a lot of research but the info is overwhelming and I cannot swim due to my issues.
My pool is above ground 5,000 gallons. I have one chlorine tablet in a floater that I keep inside a small inner tube in the center of the pool under a cover.
I keep the cover free from debris. It has small holes in it for whatever purpose. Every few days I uncover to inspect. The water is clear. I do not filter because it is covered, clean and I am not swimming. For a long time no chlorine registered. Finally it did (could actually smell chlorine when I lifted the cover). It was on the high side of being normal. But there was 0 Ph reading. In fact all other readings (using a disposable stick) were very low or zero other than chlorine. I decreased the flow in the floater and have not measured since (2 days ago).
All I want is to be able to safely swim in the pool. Just the bare minimum. Is there any advice you could give to let me know what that bare minimum should be and how I get there? What am I doing wrong? Thank you SO much for anything you can tell me. I am just really overwhelmed.
Hi Jaimie, sounds like you are very determined, and are doing the right things. Your test strip has been lying to you however. Your pool water must have some pH level, it is just not registering. So I would ditch those and buy a new pack of test strips, or a small dropper test kit. I like the Taylor troubleshooter, best $20 test kit there is… Or the AquaCheck Silver 7-way test strips. Now, one tablet is probably fine for your size pool, but could possibly do with 1/2 tablet, replaced weekly. Again I would cast doubt on your test strips, might also be lying about your chlorine level. I would also run the filter, even though its covered, chlorinated and not being used. Every day, at least a few hours, or enough run time to pump all of the 5000 gals, thru the filter at least once daily.
When using your winter algicide, must it be applied while the pump is running or can this be added during the middle of winter when the pool is completely shut down with no pump circulating the water?
Hi Doug, the algaecide will disperse effectively by pouring it into the pool, there is no need to run the pump. The only chemical that really needs to be circulated is the Stain & Scale chemical, e.g. Stain Away.
Hi.
I am pool man and I am taking care of a new replaster (april 2020) pool 15.000 galons.
Since restart the pH is always high (more than 8, measure by Taylor). I am adding little by little sodium bicarbonate weekly to increase alkalinity and today is about 100. It’s a salt cell pool. I add one galon of acid every service, the pH goes down, but in one week (maybe less, I don’t know), the pH increase again. I don’t know what to do. Help me!
Ricardo, you are doing the right thing, and this is perfectly normal for new plaster pools. For the First 12 months only, new plaster pools give off high amounts of carbonates and bicarbonates, and this elevates the pH. Happens to every new pool or new plaster. Around the one-year mark, it starts to stabilize. Just keep adding the acid. PS – using trichlor tablets can help reduce the problem, as they have a low pH level, very low when compared to liquid chlorine, or a salt system, both of which will raise pH levels.
30000 gal pool
ph 8
alkalinity to low to read
copper 2ppm
everything else normal range
added baking soda and ph decreaser
will need to add more baking soda
why would alkalinity be so low?
had water sample checked at 2 different places
thank you
Alkalinity can drop to near zero if large or continuous additions of acid are made to control (lower) pH level. Just keep adding alkalinity increaser and pH decreaser, separately, until you can get the alkalinity up to 80 ppm, and pH down to 7.6. It may take some time, and many adjustments over many weeks, but keep at it!
Hi
I have refilled my lay z spa and ph is low, alkalinity is high. If I use ph minus and use the blower to raise ph, how long do i need to keep the blower on? Roughly perhaps! I don’t want to waste test strips. Thanks
Hi Robin, I’ve no idea, but it is not that effective for large movements in pH level. I am not aware of any data that gives advice on how effective aeration can be in raising pH. I would be interested to know, but in any case I believe that the most you can expect is a couple of ‘clicks’ on the pH scale. From 7.1 to 7.3, for example, and it probably would take several days, maybe a week? Just a guess. Please let us know how it goes!?!
Can I add bromine starter and ph balance at the same time I fill spa
Hi David, sure thing, I would add the pH balance first, and then within a few minutes, you can add the bromides.
I’m curious I have been having issues balancing my ph and alkalinity in my hot tub that uses chlorine for sanitizing. Right now my ph consistently been testing at 6.4-6.6 but my alkalinity is about 110-120ish sometimes it is higher. So I have tried lowering the alkalinity down to 40-60 and then I try to raise them both together with ph+. But for some reason the alkalinity level goes up but ph stays at about 6.4-6.6. Now this may be a stupid question but I am going to ask anyway! Does the the same principal of using air from an air compressor to bring up the ph without raising the alkalinity apply in a hot tub as it does for pools?
Hi Trinity, yes it does, you can raise pH levels by aeration, which can be done many ways, but one way is by laying an air hose in the bottom of the pool, (tub) with several air outlets if possible, to bubble air thru the water. If your spa has a blower, that is probably the best and easiest way to aerate the water. Water falling and splashing is another, from a fountain, spout, neck jets or deck jets, and such water features. Let me know how well it works? Also, consider cross-checking the pH test with another test kit, or pool store test.
1. Pool pH looks ok with 7.62, but alkalinity level was under 80 ppm
2. What are the relations between pool water alkalinity and hardness?
3.Is zero hardness level possible in an above ground pool water? If so, can it be safe for my pool water chemistry balance and for safe swimming?
Alkalinity and Hardness are not related very much, only in passing (how ya doing?). Zero hardness is technically possible, but not likely and not good for the pool or water balance in general. Alkalinity is OK a little under the generally accepted minimum of 80 ppm. Calcium should be 150 ppm minimum, and best around 200 ppm. If too low, the water is too soft, and foaming and cloudiness can result, and damage to the plaster (concrete pools).
I have a 28,000 gallon pool. The salt cell is prone to playing out. My PH is constantly high. The minute I adjust it, my Alkalinity falls to the floor. Seams like no matter what I do, I cannot satisfy both PH and TA. Vinyl lined. 2 years old. No issues until the scg started plating out. Any help would be appreciated.
Hi Mark, saltwater pools tend to have a high pH due to the constant addition of sodium hydroxide as a waste product of the chlorine generation process. 3″ Chlorine Tablets on the other hand, have a low pH and will drive your pH to the perfect 7.3 range, where chlorine is more effective. Same thing with using liquid chlorine, which has a pH of around 13. Switch to tablets and problems with pummeling your alkalinity in an attempt to lower pH levels, will go away.
Ihave a salt water Annie ground pool that is 12500.My ph is 6.5 and alkalinity is 180ppm. How do I raise my ph without my alkalinity going through the roof?
Hmmm, tough situation. Can you drain half the pool and fill up with low alkaline water? You would then also need to add more salt, but it could be easier than doing the dance of up/down/up/down/up/down… which is my only other advice, really…
I have a 12,000 G, pebble surface, salt water pool that’s about 5 months old. I’m always battling high PH & Low Alkalinity. At first I tried dosing the pool with acid once per week to control the PH, but in a week the PH would go way too high (around 8.2 or more). So, I’ve been trying to dose the pool daily which is working great to keep the PH in range, but now my Alkalinity levels are falling too quickly. Do you have an idea for keeping my PH & Alkalinity more balanced? I’ve tried running my salt at a lower percentage but that just results in clorine levels that are too low. thanks!
Hi Kerri, you sound absolutely desperate! :-0 Don’t worry it will all sort itself out in another 5-7 months… new plaster or pebble is like that, it gives off a high pH residue for about a year, and then it will be fine. Now for the low Alk issue, of course this happens when you constantly add pH down or acid, the alkalinity gets pummeled. So what to do? Add Alk Up, aka sodium bicarbonate, to counter-balance, which will also raise the pH, but not quite by so much. For 12K pool, I would plan on adding 2 lbs per week of Alk Up, for the next many months, so you may as well invest in a 50 lb bucket. 🙂
I have a 22K-23K gal in-ground salt water pool with an auto-cover (West TX, lots of heat and lots wind/dirt). My free chlorine has been about 7.5-10.0 all summer, and my total chlorine has maintained the same levels with very infrequent deviations of the combined chlorine. I’ve turned my chlorinator down to 20% for the last month. My pool would need about a pint (16-20 oz) a day of muriatic acid to maintain 7.2-7.6 pH, but that has slowly but surely reduced my alkalinity to 80-90. If I slide the pH back to 7.8 then it wants more than the approximate pint of acid, which then pushes my alkalinity to the low end again. My calcium hardness is on the low end, but everyone here tells me not to sweat that (trying to give you everything). Am I overthinking the alkalinity if I can keep my pH in the sweet spot? No one has complained, but I don’t like the higher chlorine reading. Any combo solutions that don’t involve draining the pool?
Hi Richard, yes to getting the chlorine reading down much lower, 2-3 ppm is where I like it, and I think that if a pool is operating fine at alkalinity of 60-80 ppm, then that’s okay. You may find your pH more erratic at a lower alk level, requiring more pH adjustment, which drives alk even lower, you know. If it reaches 60 ppm, I would add a boost of alkalinity increaser, about 5 lbs, and that should not bump the pH too much, maybe just two clicks.
I have an 18,000 gal in ground pool. My Ph has only occasionally needed adjustment downward over the years, and my alkalinity has always been high. Not a problem until this summer alkalinity still 180, but PH has dropped to 6.?
Per your recommendation I’ve “pooled my acid” and gotten the alkalinity level down to 150, and PH still 6.? Aeration didn’t make any difference in PH, so now I’m adding soda ash and have PH back to 7.2, and in the process of getting it back to proper level.
So, I’m thinking PH is more important than alkalinity? And do I just continue to go back and forth adjusting both? Will it eventually level out a little? HELP!
Hi Liz, keep at it yes – pH is indeed more important, alkalinity affects pH, but if the pH is good and stable, we don’t need to worry about alkalinity, even when it is outside of the recommended ranges.
I have an intex 12×30 easy set round pool with 1485 gals, a new set up with filter/pump included. I tested the water after initial shock of half a pound of chlorine granular pool shock (chlorine a little high, hardness low and acid low… pool store tested the next day and said all that I needed was hardness, treated with hardness 2lb 10 oz… now the next day(day 3) ph at 7.4, chlorine 1ppm, acid same, and alkalinity 40ppm, what should I do now? Is it safe to swim?
Hi Dianna, yes safe to swim, but the chlorine won’t last long, 1 ppm is the absolute minimum. Use a 3″ tablet to maintain daily chlorine level of 2-3 ppm, and then use shock for weekly boosting, or if you see algae, or cloudy water, or if chlorine level drops below 1 ppm. Raise the alkalinity with about 12 oz of baking soda (sodium bicarbonate), which will also raise your pH level slightly.
I’ve been using liquid chlorine in my 25,000 gallon in-ground, vinyl-lined pool due to trouble with high cyanuric acid when using tablets. Of course, this means I’m constantly battling high pH and having to balance with muriatic acid every few days. Is there any more practical way of keeping my pH balanced other than using tablets or constantly adding acid?
One of my favorite wasy to reduce the buildup of cya, is to add a mineral purifier, like Nature2 Express or a Frog unit, and then reduce your chlorine tablet use by half, running a chlorine level of around 0.75, with a low cyanuric level of 20-30 ppm.
Hi, have an in ground pool. First up my FCI is low (0.5), what is the best way to raise this? It was around 1 the last time I tested it. Secondly my Alk is between 80-120 and pH is 7.2 but my pool is still cloudy. Any suggestions on how to get it back to looking clear? Many thanks in advance
To raise the chlorine level, add more chlorine. To clear the pool water, add more chlorine, and run the pool filter longer each day.
Hi Davy,
I have a 20k gallon salt water in ground pool. My alkalinity is at 140 and ph at 7.2. Usually my ph is between 7.6 and 8 and I add morainic acid every week or so to keep the ph in good range, while my alkalinity has always been around 100-110. For some reason it is up to 140 and I have trouble bringing it down with muraitci acid, while keeping an eye on my ph. I don’t want to go lower than 7.1 or so. What’s the best approach on how to bring down my alkalinity by 30 ppm? Without crashing my ph?
David, the best way may be to first raise your pH up to 7.8, then add acid to drop the alkalinity. The pH will also drop so raise it up again, and if needed, add acid to drop the alkalinity further.
Hi! I have above the ground Intex 18ft c 48″ and the pH and alkalinity are both very high. I already poured the complete container of pH lower and it keeps being high. Its a saltwater pool. What can I do now? Salt level is good.
Just keep at it – salt pools tend to rise in pH due to the hydroxides created. Get a good pH and alkalinity test kit like the Taylor Troubleshooter.
I cannot get my ph to go higher. I have used 4lbs of soda ash and nothing. I run a swim school so there are kids in the pool all day 5 days a week. And because of that I put in 1lb of chlorine in it everyday. But I just can’t get the oh to go up. Alkalinity is fine, just low ph I can’t get up
HI Shannon, tablets (chlorine) have a low pH and will push the pH down. How low is low? If it is 7.2-ish, that is fine in my book – keeps chlorine very active. And the pH of the eyeball is about 7.3, so also good with low-er pH. If you are below 7.0 however, then yes it is good to raise it, to protect the pool plaster and other soft and shiny products in the water. I would add more soda ash! If your alkalinity is very high (over 125 ppm), it could be making it hard to change pH, but if you keep at it, it will rise up. Love what you are doing with the swim school! Must be very rewarding!
I have a brand new in ground pool. My Cholorine and PH Levels are both high. Need help@
Hi Gene, the chlorine level will come down on it’s own, but if you have tablets in the floater, remove the floater, or if you have a chlorinator, turn the dial down to zero. For the high pH, it’s important to lower it, so chlorine is effective, so algae won’t grow, and to prevent scaling. New concrete pools have high pH for about a year, and salt pools also have high pH, and pools that use liquid chlorine also will have high pH. To lower high pH, to a more basic range 7.2-7.4, use an acid, either liquid acid or dry acid. Be careful not to overshoot the level, and know that when lowering pH, you also will be lowering alkalinity, so you will not only need a bucket of pH decreaser, but a bucket of alkalinity increaser
Hello! I have a 13,000 gallon above ground pool that I have been trying to open. The water was cloudy and dark green and chlorine, pH, alkalinity and stabilizer were all at the lowest on the test strip. I added over the maximum recommended amount of pH up and alkalinity up multiple times but pH won’t move. The alkalinity increased slightly but still low. I have tried to vacuum debris out of bottom of pool and have gotten a lot out but I’m sure there’s more down there. It’s so cloudy I’m unable to see what’s at the bottom. I have tried shocking it multiple times but the chlorine level doesn’t seem to ever go (not even a little). I put 2 bags of chlorine stabilizer in the pool but the cyanuric acid level has not budged either. Am I doing something wrong? Should I try blindly vacuuming the bottom of the pool before trying to balance it? Or maybe the chemicals keep going to the bottom so should I trying stirring up the bottom? The water has lightened up a little but it’s still very green and very cloudy. Any recommendations? Thanks!
Hello, yes try to blindly vacuum the pool to waste, to remove the material on the floor. Check that the pH level is below 7.6, if not, add an InTheSwim brand pH decreaser. And then add lots of InTheSwim brand chlorine shock or liquid, until the water turns a blue/grey color. And perhaps most importantly, run the filter all day and night, 24/7. Adding an InTheSwim brand clarifier will also help. 😉
Hi really hope you can help, we have a 6000litre above ground 12ft best way pool, it runs 24/7 with a filter and a heater. I use dip sticks and chlorine, ph and alkaline all low. I added 400grams of dissolved PH into it lastnight and made it cloudy and this morning retested and same result with very cloudy pool. I always have floater in there with around 4 tablets but don’t understand why all my levels are low and what to do to help it, kids desperate to get in the pool but not sure how to fix it thanks
Hello, adding pH increaser can cloud the pool water, that is normal. For the low chlorine level I would recommend shocking the pool with a triple dose of chlorine, adding 1 lb of granular chlorine to the pool in the evening. Test again in the morning, you should have a high chlorine level still. If the pool is very sunny, it could be that it is burning off most of your chlorine. adding stabilizer, aka cyanuric acid can help., just 12 oz or so is all that is needed, for 1500 US gallons.
Just set up an 8′ X 30″ small intex pop up and filled it with our softened well water. The water appears to be clear, but yellowed the white in the “liner.” Our water is heavy iron (of course) and chlorine 0 (of course). The test strip is off the chart for ph and alkalinity (of course). I don’t have a pump. This is the same water we bath and shower in. I set a couple of tablets afloat. Is there any reason not to get in, and does the float come out when we get in?
Hi Scott, no filter will be a problem. Even chlorinated, it won’t last longer than a week or two. The pH should be lowered also to around 7.2. If it is 8.2, only about half of the chlorine will be active, and also high pH makes algae grow faster. Tablets in a floater is a good idea, but you will also want some granular chlorine or bleach, to boost it up high and keep it high, around 5 ppm. You can leave the floater in the pool while using the pool, or remove it, does not matter really. Get a filter/pump if you can find one. If not, brush the pool and skim the surface twice per day, for a little circulation. Test daily. keep chlorine high and pH low. Drain when it starts to look cloudy.
I have an 800 gal. indoor soaking tub covered and kept at 91 degrees.
The alkalinity was at 0 so we added a few pounds of baking soda which brought it up to maybe 50 or 60, but then we noticed that the pH was very high way over 8. So if we now add muriatic acid to bring the pH down won’t that lower the alkalinity again? How do we keep from going back and forth on that? thanks. ralph and sherry
Hello, you just go back and forth as needed. Sometimes overshooting each a little bit, see-sawing until you get it right.
Hello! I have an 800 gal stock tank pool (8ft x 2ft). It is galvanized steel. We live in south Texas where it is very hot. We have a sand filter system for the pool Our water is typically very hard. We are constantly struggling with the oddest thing: low PH (about 5-6) and high alkalinity (180). What should we do? Some days it’s nice and clear, other completely murky. I skim it every day and we keep it tarped at night to avoid debris. I usually shock it once a week and we keep a chlorine floater with 2 1-inch tablets at all times. I swear I’ve added every chemical from every blog and I can’t keep it consistently clear. Any thoughts?
I think I would check the pH/Alk of your fill water, is it the same? Any close neighbors, to check their pH/Alk? I’d even think it would be easier to drain the pool, and refill it with water that you haul from across town in 55-gal drums. I don’t know what else you can do, other than aerating the water, to raise pH a little bit, or keep adding soda ash pH increaser.
Excellent blog! I have a 33,000 gallon vinyl pool. Alkalinity is 110-120, ph is 6.8-7.0.
I’ve been adding ph increaser and just turned up the return eyes. The ph has not risen after two weeks. The pool is clear. Should I leave it alone? Add baking soda? Something else? Thanks for your help!
Dave
Hi Dave, as you know, 7.0 is neutral and 6.8 is verging into acidic territory, which you want to avoid, to protect your liner and other soft/shiny materials in the pool. It would be good to add more Soda Ash or pH increaser, to get to 7.0-7.2 range. Also ask yourself if you can trust your test method, if using test strips, they can be hard to discern such small amounts, and the average strip can be off by 10% or so…
Five of us pool owners on my street run the Nature 2 Fusion chlorine tablet dispenser and mineralizer. The device makes our pools sparkling clean. We have no water issues. I’m simply reporting on an anomaly with our test strips.
We’ve noticed we can’t get a reading on the PH and Total Alkalinity pads on our strips. They simply do not react. We’ve tried strips from different companies. Same result.
As a test, two of us pool owners, added substantial amounts of Baking Soda and similar products to boost the Alkalinity in an effort to get a reading on the test strips. In the process, the pool water at both houses became cloudy and slime and scum formed on the stairs and around the liner, clear indications of over boosted Alkalinity. A slight level of Alkalinity was detected on one of the strips at this point.
It appears that the test strips we are using are being chemically interfered with by something either in the chlorine or in the mineralizer.
Have you ever experienced this before?
Hi Robert, I have not heard of such a thing before. I would not expect the chlorine or minerals to cause test strips to not display a reading for pH or alkalinity, they simply are not related to each other, but wholly independent and can think of no reason why either would affect your testing. Something else must be going on. You may wish to contact the manufacturer of Nature2, which is Zodiac Pool, (now Fluidra) and see if they have any advice or comments. https://www.fluidrausa.com/en/support
Hi There,
I have a new 3600 gallon endless pool indoors. I can’t get the alkalinity down lower than 200 (started at 340). I have been using Lo n Slow by bioguard. Current I am sitting at pH of 7.2-7.3 w alkalinity at 200, free chlorine at 2 and calcium level around 600. What are your suggestions for lowering it. What should be my goal with that? My
Pump is external to the pool in a closet. I also have an attached UV filter. It is a vinyl lined pool
Thank you kindly,
Hi Andrea, if your pH sits at 7.2, an LSI calculation would want your alkalinity at 150 ppm. Or, if you keep your alkalinity at 200, drop the pH to 7.0 – that would also be in balance. You can play around with the numbers here: https://www.pentair.com/en/knowledge-base/pool-spa-equipment/pentair-pool-calculators/saturation-index-calculator.html
I have a 30000 gal plaster inground. The std cleanup after opening is 2 weeks. I try to go according to all the chem levels but seem to usually settle at 7-7.2PH & 150-170TA, with CH1-3. It’s been mainly clear all season with sometimes a slight cloudiness. Are these readings ok or should I continue to add 5lbs soda ash twice a week? I use 15-20 3” trichlor tabs a week; water has been over 80 degrees most of the time.
Hi Tim, the high alkalinity is making it hard to raise the pH level. 150 ppm is not necessarily going to give other problems, altho you may battle occasional cloudy water. The low pH level should also be fine, but if you slip below, into acidic range, it could etch the plaster over time, so keep a close eye. What is most curious is “15-20 tabs a week”, really? That is a LOT, most 30K pools would use about 6-7 tabs per week. I would do a very heavy shock, a ‘triple-shock’, or 3 lbs per 10K gals, you must have some invisible consumers of chlorine, such as nitrates or ammonia.
Trying to raise ph put 5lbs in 1 lb at a time circulating 24hrs for past 2 days, notice ph was turning deep blue when I put it in pool. Pool water now cloudy with blue bottom of pool. Vacuumed it, still cloudy and no ph increase. Turned eyelet up on discharge overnight. Nothing! What do I do now, keep making pool cloudy by putting more ph in? 10,000 gallon pool. And why did increaser turn blue, backwash pump and blue sediment comes out.
Alan, that’s a new one for me! Soda Ash (pH increaser does tend to cloud pool water, but turn it blue?) I found a reference online to the same problem, and it was suggested that it could be metals/minerals, which makes sense. Oftentimes, a large adjustment to pH can cause them to come out of solution. Blue would tend to be copper in the water, precipitating out of solution. Use a sequestering agent, aka Stain & Scale chemical, there are many like MetalFree, ScaleFree, or our Stain Away, which will lock the metals back into solution, or chelators like CuLator, a pouch that will absorb the metals. These chemicals require frequent re-ups, because they degrade with sun and chlorine. Afterwards, continue adding pH up.
Have a 30k gallon in ground pool with sand filter. Have used chlorine tablets all year. Ph is so low won’t register on the dip sticks but guessing maybe around 6 after 6 pounds of ph increaser…severely low alkalinity as well. Do I just keep pounding ph increaser in it?
Yes, keep adding pH increaser, which will also raise alkalinity. If you get to a point where the pH is OK, but alka is still low, you can add Alk Increaser, which won’t raise pH as much as alk (but still some).
I have a 300 gallon spa – the problem is that the tap water I use to fill it has a starting PH of 8.1 while TA is only 40mg. I’ve verified this both by taking it to a pool tester and from the water authority quality reports. I struggle to get any sort of balance in my pool – If I bring the pH down the TA crashes to about 10, but the PH starts to creep up again straight away, but every time I add even a tiny amount of dry acid the PH crashes. If I try to raise the TA to 80, the PH climbs to 8.4 and the water is cloudy. So I can’t seen to get any buffer in this spa, any ideas?
HI James, settle on 60 ppm for alkalinity, and something between 7.1 and 7.7 for pH. It’s ok to be a little bit off the mark in most cases. Run an LSI calculation, using the Pentair LSI tool –
pH is 7.2, alkalinity is 150. How do I reduce alkalinity without crashing pH? Pool is very cloudy. Inground-22,000 gallon.
Hi Mark, you don’t there is no chemical that won’t adjust both at the same time. But if you want to stay at 7.2 and 150, as long as it isn’t giving you problems, you have my permission! 🙂
Help! My pool has been crystal clear most of the summer, then we got a bunch of rain now my pool is cloudy. I have shocked it, used clarifier, tried to lower alkalinity, with no changes. Also I have been running my pump 24/7 . My PH is on the lower side but the alkalinity is still too high. What can I do to clear it up?
Hi Lisa, you may have a high phosphate level in the pool possibly, or perhaps your cartridge needs to be replaced, if you have a cartridge filter. Your filter may have other issues too, perhaps bypassing water without filtering all of it. Don’t worry about the alkalinity, but keep chlorine high and pH low.
My hot tub ( fresh Water) alkalinity is high ph is good can I treat with vinegar
Vinegar is a pretty weak acid, and it will make the tub smell funny. I would recommend using spa pH decreaser, or muriatic acid, not vinegar.
Help! First time above-ground pool owner here. It’s a 15 by 48, intex easy set.
Two things: since I shocked the pool, about 10 days ago, I haven’t been able to get a chlorine read, even though I have chlorine tabs in the floater. The pH is okay, the total alkalinity is just below the line.
That’s one situation. Then, someone went in the pool wearing her clothes — brown pants, maroon shirt. I know. Since then, the water has been a little cloudy, but the filter keeps filling with a brown/maroon stain, and I don’t know whether it’s algae, or it’s just taking a long time to filter out all the brown fibers that were left behind. I’ve been changing and washing out the filters, there’s not a lot of “gunk” in them, but they are staining.
I get in and clear out debris every day, and I scrubbed the sides yesterday, because I had noticed a slight scaly/slimey feel.
What can I do to make the water clear again?
Hi, try using a Clarifier, per label instructions for the cloudy water. For the chlorine reading, shock the pool again, but use a triple dosage, per label, and maybe you need more chlorine tabs, and/or add 1 lb of cyanuric acid, aka stabilizer, to protect chlorine from the sun.
We have a 5000 gallon above ground pool. The pH is 7.2 today with Alk of 150. Seems on the edge of ideal. What should be do to help edge this back into range. We have saltwater converter. Thanks!
First I would use pH increaser, to bump the pH up to 7.8, then add an acid to lower the alkalinity to 120 ppm or so, then you may have to raise pH again.
We had a freshly filled pool (39600 gallons) about two weeks ago. Our PH was 7.2 and our Alkalinity was 240. I have used about 12-13 gallons of Muriatic Acid over the last week to lower the Alkalinity, not all at one. I have put in about two gallons a day spaced out. I have dumped the Acid in the pool with the pump running. My Alkalinity was still about 120-130 and my PH had dipped to about 6.5. I put in about 4 lbs of PH up by mixing it in a bucket of water and putting in pool. My Alkalinity shot up to about 180-190 about an hour later. So then I put two gallons of Muriatic Acid in pool at once. I am not sure what to do now.
Give another round, and maybe a third round, and you might get there. If this is a plaster pool, pH will rise on its own naturally, so you can cut back on the pH increaser. It’s ok to have alkalinity a little higher than recommended, it can go up to 150 ppm in many cases without a problem, as long as you have a decent pH of 7.2-7.6
I have an above ground 24x24x52”, is there a way to lower ph without lowering alkalinity?
No, there is no way to do that. The same chemical (pH decreaser) lowers both alkalinity and pH.
20×40 in ground pool. pH was 6.2. Alkalinity was Zero. Added 8 boxes of baking soda… pH rose to 6.3 and alkalinity was about halfway to normal. Added 8 more pounds and also added 6 pounds of soda ash (not at same time). Seem to be having a helluva time getting the pH up. Any advice other than more soda ash? Also cya at 111 and normal is 0-100. Pool store says anything under 150 is safe.
FC is good.
TC also good.
Any advice on all of this appreciated.
Just keep at it Jason. If your tap water (pool fill water) is better quality pH/Alk, you may consider draining half the pool, and refilling, to lower the Cyanuric and help the pH/Alk situation.
Hi, I have a 14000 gal. in ground pebble tech pool. Im struggling with low alkalinity and high PH. Can’t seem to raise TA without PH going way up. Im fairly new at this. I use baking soda to raise TA then Acid Magic to lower PH which puts my alkalinity back down defeating the purpose. I tried the bucket trick and the bucket just floats. Help !! TA is runs around 65ppm and PH is 8.0.
Hi Robert, just keep at it – more important to get pH down below 7.6, if alkalinity is a little off, less of a problem. I see the problem with the bucket trick yes, maybe a heavy stone or brick is needed.
first time pool owner in small rural area. One year old above ground (6649 gals) saltwater pool system. Per pool store where purchased we should regularly using Bioguard lo n slo for alkalinity and ph. Water is clear but both are high per test strips. Also have started having sand in the bottom of the pool. What is that? Know nothing about pool or chemicals.
Hi Ray, Lo & Slo is a granular pH and alkalinity decreaser, similar to our own, and if your alkalinity is much above 120 ppm, or if your pH is above 7.6, adding some of the dry acid will make your water more basic, lowering the pH and alkalinity at the same time. Add one pound of dry acid, per 10,000 gallons, to move pH from 8.0 to 7.5, for example. If you have sand in the pool, if it is widespread, it may be blown in or brought in on feet or swimsuits, or if it is right below the returns, in a small pile, then it could be from a sand filter, if you have one. If it happens only after backwashing, and then stops, no worries, but if shooting sand all the time, then it’s likely a cracked standpipe or lateral(s).
My pH is controlled by CO2 and stays between 7.4-7.6 all of the time but my TA will at times go above 200. If the pH is normal doesd the TA level matter?
Good question, and No is the answer, if you have a good pH level, then who cares about TA? Well, in some cases, it may throw the pool out of balance, and can contribute to scaling, when conditions are right, or lead to cloudy water when other conditions prevail. But if you are not developing calcium scale or cloudiness, no worries then.
Hi. I know you must be tired of answering these questions, for that I am sorry.
We have an Intex above ground round pool. It hold apron 4440 gallons. When testing my water The alkalinity and pH were both high. The saying cyanuric acid was extremely low and so was the free chlorine and the calcium hardness was at the low end of acceptable. I used ph decreaser and now my ph is at 6.4 and my alkalinity is on the high end of normal. I am not sure what to do to correct the imbalance. If I increase ph wont the alkalinity also increase?
I got calcium under control. Both free chlorine and cyanuric acid are staying low even after 2 shocks.
Hi Valerie, for the pH to rise, you should add pH increaser, and yes, it will also affect the alkalinity, so you will need to adjust alkalinity again, and then perhaps raise pH again. I wish there was a simpler way to deal with low pH/High alk, but there is not, just keep correcting and recorrecting. As for the cyanuric, if your pool is sunny, you could benefit from the protection from UV it provides, and it can save money on chlorine expense. For your pool size, add 6 oz of cyanuric acid for each 10 ppm increase – best range is 30-40 ppm. Problem is, 5 lbs is our smallest size, at $30. It may be better to cover the pool, to block the sun, lol. Or just do without, and add another tab to your daily chlorination routine.
I have a 18×36 18,000 gallon inground pool. My chlorine is at 2.13, PH is at 7.7 and Alkalinity is at 147. I am new at this so still trying to figure it out. Will the PH down work to drop all the numbers?
Hi, the pH down will lower ph and also will reduce the alkalinity level. It will not affect chlorine. Work at getting alkalinity below 120 ppm, and pH below 7.6
Hi there!
I have a 35,000 in ground fiberglass pool I acquired when I purchased my home. I have test strips to test chlorine , oh & Total Alkalinity levels. The chlorine level looked okay at 1 ppm. But the ph was yellow gold instead of red orange -low reading)
And the total alkalinity was also yellow instead of green /meaning low. I use the 3 “ tablets in the skimmer and a floating broadcasting and about a week ago I added a gallon of liquid chlorine because green algae was forming on walls slightly due to the hot weather outside and pool floats in the pool also.
What do I need to add to raise both the ph and the total alkalinity levels for safe swimming?
Muratic acid?
Thank you so much!
Lisa Kroske
Hi Lisa, you do not need an acid to raise pH and alkalinity, but a base. Start with ALkalinity Increaser and that will also raise the pH level. Get a 25 lb pail, you may need it all with your big pool, add half, retest, and possibly add the remainder. Add about 5 lbs to increase the alkalinity by 10 ppm, for your pool size.
Hello.
I have a 24ft x 52inches deep above ground round pool. So it rained yesterday and after it had stopped my husband shocked the pool last night. We checked our pool this morning and it was sparkly clean and clear but our PH and Chlorine level are both high. My husband added too much shock last night so that is why our PH is at 8.2 and Chlorine is at 3.0. We are brand new pool owners and don’t know what to do now. Somebody told us to put clear balance in the pool and we did but that did not help at all. I googled some questions and just saw that we could have used PH decrease but now we are afraid of adding more chemical to our pool. Please help.
Hi, if you are using granular shock, that has very little effect on pool pH. What you need to lower the pH, down to 7.2-7.4 range is to add an acid, or pH decreaser to the pool. Chlorine at 3 ppm is slightly high, not too high at all. But at pH 8.2, much of the chlorine is lazy and inactive.
I have 27×52 pool that ph and alkalinity are super high I have added gallon of muriatic acid and is still high , 17500 gallon pool should I continue to just add acid until levels come down before I shock it
Yes, keep adding more acid if it is still high. Might need another gallon, but proceed slowly if your test kit does not have an Acid Demand test.
We are new to pool ownership. Above ground pool, about 4400 gallons. Currently, our ph is 7.6 and Alkalinity is way high, now around 360. Chlorine is at good level. Have put in ph down daily for past several days, ph has stayed same and Alkalinity has dropped just slightly. Haven’t tried bucket method to put ph down in, but hoping to get idea on how to lower alkalinity since its not moving very much.
Russ, that’s how you do it, you already know how. Just add acid, The bucket method of adding acid is supposed to create a more rapid exchange of carbonates, to affect alkalinity more than pH.
We inherited a 27ft round above ground pool with a salt system when we bought this house. Still learning how to maintain it. The ph stays high but alkalinity is consistently low. I thought this rose and fell together. Which do I adjust? Thanks
Hi, you will want to adjust both. salt systems tend to rise in pH, and constant acid additions tend to lower alkalinity. So you need alkalinity increaser, and more pH decreaser, and keep making adjustments to both, until you get them in balance.
We had a ton of rain the last two weeks that lowered my alkalinity. I fixed it but then I got yellow algae. I treated it and triple shocked it and cleaned it out. Today my pool is clear! Yay. BUT, I tested this morning and my chlorine is at zero!!! My ph is a bit low and my alkalinity is reading about 60. I have chlorine tablets in my floater. How is it reading zero?
My pool is a 24’ above ground. I usually keep up on my chemicals but the storm did a number. What suggestions do you have?
Jennifer, the mantra is that if you shock it hard and the next day the chlorine is at zero again, you need more chlorine – the demand is greater than the supply, and it is still consuming it, so another shocking is a good idea, to restore order and be able to obtain a reliable daily reading from a few chlorine tablets. Maybe not a triple shock, but a 1.5-ish shock would do
Hello. I have a bestway 18×52 round pool. My chlorine is at 0. My pH is a little high (7.8) and Alkaline is at ideal level. What should I do to lower pH and not mess with Alkaline? And how do I bring up my chlorine levels? I added chlorine stabilizer yesterday.
Hi Maira, lower the pH by ‘walking the acid’ splashing it on the surface as you walk down the pool edge. This is thought to have less effect on alkalinity as opposed to ‘pooling the acid’, or slowly pouring in a quiet corner of the pool, with pump off. Bring up chlorine fast by using granular chlorine, one cup per 10,000 gallons, should bring it up to around 5 ppm. First do the acid, because chlorine is more potent at lower pH levels, then add the chlorine after waiting 10 mins.
what does walking the acid mean ? My alkalinity is to low and my ph is to high in my 14000 gal inground pepple tech pool. can seem to adjust both without effecting each other.
Yes you can’t really adjust one without affecting the other, but ‘walking the acid’ and letting it splash into the pool is thought to affect pH slightly more than alkalinity, while ‘pooling the acid’, or adding dry acid into a bucket and setting the bucket underwater in a quiet corner or step of the pool, is thought to affect more alkalinity, but it is very slight.
Help Please. I have a Summer Waves 16 foot by 48 inches. I have tried Muriatic Acid and I have tried pH Down to help decrease my pH and my alkalinity levels every time I do this they shoot back up within a day or two I feel like every two days I’m adding more PH down or M. acid. My chlorine level also fluctuates I was using liquid chlorine at first but then read that that raises your pH levels. Pool looks crystal clear except for every few days there is a dark substance looks kind of like sand in spots on the bottom which I vacuum out.
Please help. This is my first time owning a pool.
Hi Breeanna, if your alkalinity is very high, over 120 ppm, it can resist your efforts to lower the pH level. If the alkalinity is very low, you can get what is called pH bounce, where it will change for a few hours or days, then return to previous levels. Try to get your alkalinity in line, or between 80-120 ppm, and that should help stabilize the pH level.
Hi we have an 18 foot 4.5 feet deep round above ground pool with a sand filter and a salt water system. When I test the water the alkalinity is always too high and the PH seems to run high so I use PH down almost every day. the PH will come down some but the alkalinity stays high and I usually show little to no free chlorine. If I shock it the free chlorine will increase to normal for a few days. Should I continue to shock it regularly? And do I need to worry about the high alkalinity? This is our first time having a pool and I’m just guessing about what I’m doing. Thank-you for your help
Beth, salt systems will generally run a high pH level. High alkalinity resists your changes to pH, so keep adding pH down, but in larger doses, to try to get the alkalinity down to 125 ppm or less. For chlorine level, use enough chlorine Tablets to maintain a daily chlorine level of 1-2 ppm. Pool shock is used for other reasons, but not for daily chlorine level, unless you run out of tablets, which dissolve slowly to give a constant and consistent chlorine level. Cyanuric Acid, aka Stabilizer is also good to use for sunny pools, to protect chlorine from the sun, use 3 lbs per 10,000 gallons, to raise it to 30 ppm.
Had the same issue. Finally learned it was calcium dust (the powder stuff that is hard to trap.) Used flocculant to get it all settled to the bottom & then took my time vacuuming it out.
Hey , I have a summer waves 12/33 pool, my free & total chlorine levels are both 10 , but my ph is 6.2 & alkalinity is 0 . I’ve took my chlorine tablet out for two days but still no change , I’m new @ this & don’t know what to do , please help
Hi Barb, raise the alkalinity and the pH will come up along with it, they are close cousins… For your pool, assuming 1500 gals, add 28 oz of sodium bicarbonate, or alkalinity increaser. See if that hits the mark, of 80 ppm minimum for alkalinity, and brings your pH up to 7.2. If not, add a bit more. You can also use poolcalculator.com for estimating chemical adjustment amounts.
Hello,
I have a saltwater pool that from one week to the next went from 80ppm alkalinity to 0ppm alkalinity. We have no idea what could cause the sudden drop, no chemicals were added during this week and we have not had any rain. We had a tough time getting alkalinity back up. Any thoughts on what could have caused this? Myself and several other pool techs are scratching our heads, nobody has ever encountered this before.
Possibly someone added a few gallons of acid, thinking it was liquid chlorine? Could be a household member that normally does not touch the pool. Or could be a practical joke (haha :-/). It’s not easy to get to zero alkalinity, takes about 1 gallon of acid per 5000 gals of water. Perhaps testing method is not valid or incorrect?
Hi Tim, do you have any advice for raising PH when alkalinity is already high? Chlorine levels are normal but I’m putting loads and loads of ph increaser and ph is just not rising. The pattern is now coming off my liner and I think it is as a result of the low ph – it is showing below 6,8 on the scale. Do you have any suggestions please?
Hi Ethel, add a large dose, to hit the alkalinity level hard enough to drop it down to the 50-60 ppm range. Use poolcalculator.com to compute the exact qty of acid to use. After 4-6 hours, test ph and alk, and add an exact qty of Soda Ash (pH Increaser), to bring the pH back up to 7.2, and alkalinity will raise also.
My alkalinity and ph were high but the alkalinity was much higher. The pool store told me to use ph decreaser. Now my alkalinity is better but still a little high and my ph is really low. It is a 10 foot by 30 inch pool. My water is much more clear since the alkalinity came down some. I see your truck of injecting air into the pool. Does this take a lot of time or does it bring the ph up quickly? Any other thoughts/ tips would be greatly appreciated. Thanks!
Hi Megan, it does not take time so much as repeated applications, to balance pH and alkalinity, when they are ‘opposite’. Using air, to aerate the water does not raise the pH too much, maybe 1 or 2 clicks, unless you have a large aeration system of bubblers underwater, or large spray fountains.
I have good ph, but high alkalinity.
How would I bring the alkalinity down without making the ph too low?
Hi Tim, you can’t really, you have to make small adjustments to both, lower alk, raise pH, lower alk, raise pH… on and on. Or, you can just not worry about it, which I would recommend. High alkalinity is not a bad thing, per se – unless it prevents you from adjusting pH, but if pH is good… High alkalinity can also contribute to cloudy water from time to time, but usually only when very high, over 200 ppm for example.
I have this same issue. My Ph is good but my Alkalinity is max on my strips. Also my water is cloudy. First time with an above ground pool. What should I do?
If the alkalinity is over 200 ppm, you may consider draining and refilling with fill water that tests with a (much) lower total alkalinity. If not possible, then buy a big bucket of pH decreaser, and start adding large doses, but do it in a second bucket that you slowly rest on the bottom of the pool, (with pump off and no one in the pool). And let it slowly dissolve, from inside the bucket. After an hour or two, or overnight, turn the pump back on and remove the bucket. Repeat as needed. In between treatments, add pH Increaser by broadcasting on the surface, or diluting and pouring into the pool, to keep the pH from dripping any lower than 6.8.
I have a 15 x 33 summer waves and just got it up i have not done anything as far as chemicals but when i test my ph and alkalinity are both high. Please help
Hi, Use a pH Decreaser chemical (acid) to lower both alkalinity and pH. High pH levels make your chlorine much less potent, which can lead to problems, so you are right to address it, the best level being 7.2-7.6
New pool 10000 gallon, 20×52 round. Above ground. We have well water. Filled pool now can’t get it balanced. PH and alkaline high. FC ok, I bought muriatic acid and over 3 days added a gallon. Still PH and alkaline have come down some. Still cloudy!
Chlorine level is not high but it’s not too low either I don’t think.
What next?
add more acid… and look into your filtration. Cloudy water can be a filter problem, just as often as it can be a low chlorine problem. Low pH, High Chlorine, and effective filtration, will keep the pool cleaner. Are you running the pool filter 24/7? If you are running it for 6-8 hours daily, that’s not nearly enough. You can also use a Clarifier, which will help trap the small particles.
Have used 2 gallons of acid now. Cleaning the Type A filter like every 2 hour. Shocked it today. And I made a filter with the cotton out of a pillow, hanging over the return flow inside the pool. Whatever is still in the pool is like powder. It just come right back into the pool. Ph is lower but alkalinity is still 240 or so. Water is milky.
When we vacuum it whatever this is the filter won’t hold.
I am going to try one more time with the acid. Seems like the alkaline just is stuck. Comes down just a bit but goes right back up.
The return filter is a good idea, we have a product called the Slime Bag, which is a fine mesh bag, like a small pillowcase, that connects to the return to act as a secondary filter. Another idea is to plug the return line and disconnect the return hose from the pool, and vacuum to waste, to eject the vacuum water out of your Intex pool.
You might have a torn filter grid. Same thing happened to me. Had to replace 2 grids. That powder that you mentioned might be DE or other filter media
Hi so I have a 15×48 bestway/intex pool metal frame
It has been cloudy for almost 2 weeks now
I did the shock and the chlorine shot up high like it should but it’s still cloudy and my ph level is still low I have been adding ph+ daily and no change We do use the chlorination with the 27 small tablets in it weekly but still fighting this pool
Please help I want to get the water clear
Hi Kevin, don’t worry about the pH level, low is good, as long as it is not lower than 6.8. Are you running filter 24/7? Please do. And also get some pool Clarifier, and add weekly, following label instructions. If your filter cartridge is not new, replace it – they only last a few months, before they lose ability to trap small particles.
Hi. I have a vinyl salt water pool. Usually we have to adjust for high PH but recently the alkalinity has been very low (60s). We typically do not shock as numbers are good from week to week except for always pulling PH down from 7.8-8.0 with Lo & SLO weekly. Any idea why the alkalinity is not holding? Last year it was pretty steady (90s-110).
Hi Dana, saltwater pools do typically have a rising pH, while tablet chlorine pools typically have a falling pH, both due to the pH level of the sanitizer. As for alkalinity, it can of course be lowered by pH decreaser, which may have affected it. Possibly there was some other pH reducing chemicals used this year, or a lot of low pH rain can do it as well. Get some Alk Increaser, sodium bicarb, and raise it slightly, try to get to 80 ppm.
We have a 5,060 gallon intex pool. Water is cloudy. Test strips say free chlorine 3 ph8.4+ alk 240 cyanuric acid 0. I bought PH DOWN AND CHLORINE STABILIZER. Not really sure how to use this, which one I should put in and really how much it will take to get back to normal levels. How long do I wait for it to be normal again? Can I put both chemicals in at the same time? Mostly all sun pool.
Hi Page, is your tap water of better quality? Test your garden hose water, and if the pH and alkalinity are much lower, you may be better off replacing some of the water. But to correct it chemically, you can, although such high alkalinity will resist changes to pH level, so you may have to treat many times. Add 3 lbs of pH decreaser, or 20 oz of muriatic acid and retest alkalinity and pH, retreat if needed. For the cyanuric, add 20 oz. to reach 30 ppm. Dissolve it first in a bucket of hot water, let it stand for an hour or two, then pour into the pool. This will protect your chlorine from the sun, and side note: cyanuric ACID will not affect your pH or alkalinity level.
I have a 10,000 gallon pool. The ph is 6.4. Alkaline says zero. I have added 36 ounces of ph up but the ph level or alkaline level hasn’t risen at all? What to do? Thanks
Add More! pH increaser – what else can you do? 🙂 Also be sure that the test method you are using is reliable. Maybe a cross check with a pool store or friend that has a pool, or a different test kit.
So I added more ph up. A total of 4 lbs in 2 days. Chlorine is good. But ph still at 6.4 with 000 reading on the alkaline. We use one of those electronic testers. If I add more, in what increments? Thanks again for your reply
Sarah, I would get a different test method. I am not sure if it’s possible to have zero alkalinity with a 6.4 pH (maybe?). You can get some inexpensive test strips or a pH/Cl test kit to use as a cross-check.
I have an Easy Set Pool 3638lt … it had high PH and high Alk … I added some PH reducer which has brought down the PH and now maybe too low but the Alk has not reduced, what should I do?
Plus I have added x2 Chlorine tabs to a floater to try and raise the zero Chlorine levels but this has not moved either, how should I raise this level?
Many thanks in advance
HI Billy, don’t worry so much about alkalinity, but keep a low pH in the 7.2-7.6 range. Get some bleach or granular chlorine, keep it on hand to raise the chlorine rapidly, in cases like this, and for shock treatment, every week or two. also, you may want to add a small amount of cyanuric acid, aka Stabilizer, to protect chlorine from the sun.
High TA and PH –
TA 200 and PH8.2 –
chlorine 0
My understanding is to treat the TA and PH prior to the chlorine issue, is that correct?
I have used AlkaMinus (sodium Hi sulfate ) recommend dose of 4oz for my 2500 g pool. I let it sit for a couple hours, no change – treated again let is sit a coupon hrs no change — what do I do next. Do I need the pump off and does the sodium bisulfate need to be dissolved in a bucket first? And neither of this i did.
Is it safe to swim in while trying to regulate this , not while chemicals are being added but in between treatments ? Or how long do we need to stay out of the water after the chemicals are added – I’ve seen recommendations from 30 mins to 6 hrs ?? Help please
By the time you figure out all these questions, your pool is gonna turn green! 🙂 It is best to lower pH first, before shocking the pool, or adding chlorine, because chlorine at a pH of 8.2 is very sluggish and inactive. But, your high alkalinity is going to resist your efforts to lower pH, providing a buffer to the pH. Nonetheless, keep treating with the pH decreaser, and start adding chlorine right away. For staying out of water, the only chemical that it really applies to is shock chlorine (granular chlorine), wait for 6 hours or so. Totally safe to swim now.
Help!!! I have a 15×48 above ground pool water has not been clear in 2wks PH and TA were high have added ph lower granules in last week no change pool store advised muriatic acid instead have added almost a gallon ph has lowered a bit but alkalinity still high. Clorine and stabilizer good.Water still cloudy dont know what to do help!! Thanks in advance!
Hi Tami, keep adding pH down, dry acid or liquid acid, each day, to continue to lower it to 7.2-ish. The high alkalinity is buffering the pH and making it resistant to change, but if you keep at it, you will tame the beast. For the cloudy water, probably not related to the pH/alk – are you running the filter 24/7 to try and clear it up? Your filter may be to blame, if your chlorine level is good. Most Intex pools have very small filters that are not so great at filtering very small particles. Use a Pool Clarifier chemical, and unless this is the first year for your pool, replace the filter cartridge, they only (barely) last one season.
We have a salt pool. Our alkalinity is high(163) and our ph is low(7.1)
The result is no chlorine. Would the bucket trick be worth a try?
Hi Eric, I don’t think the alk/pH is causing a no chlorine issue, you may have a chlorine lock from high cyanuric acid (Stabilizer), or lots of nitrates in the water. Have you tried a triple-dose shocking? That usually cures it. For the pH, you could add a floating fountain or develop other aeration to naturally increase ph a little bit. And yes do a bucket method when using acid to lower alkalinity. And, test your tap water and if the quality is much better, you can drain/refill a portion (and add new salt).
Hello,
I was battling a high alkalinity and high pH issue. I had to add a lot of muriatic acid over the course of a week to finally get the alkalinity down. However, the pH would decrease along with the alkalinity but then the pH always shoots back up. Now the alkalinity is actually a bit low at 70ppm (I think that is too low?) and the pH is just barely below the upper limits and I’m sure the pH will once again get to high in the next few days. I can’t seem to get the pH where it needs to be and stay there without the alkalinity being too low. Will the bucket trick really work in this situation or should I add some alkalinity increaser and then decreaser and back and forth until they find a balance? Thanks! P.S. This is a very large salt water pool and the chlorine tends to run on the high side which I am working on. Not sure if high chlorine is causing an issue with the balancing issues.
Hi Ryan, no issue with the high chlorine, but salt pools do tend to run higher pH levels. You can shoot for 7.6-7.8, but lower it before it gets to 8.0. and for alkalinity, if it wants to run lower, in the 60-80 ppm range, that’s OK too. Some pools just have an equilibrium which is slightly outside of normal recommended parameters, but it does not mean it’s technically out of balance, or that problems will result.
Thanks for the response! I recently decided to take care of my pool myself (like a year ago) and I did not appreciate the complexity of the chemicals nor the need to adjust weekly. I thought because it was a salt water pool it had little need for that. I know, VERY naive. Well, I finally took the time to research everything and what I found is that my pool was low in salt, but that’s okay because the salt generator wasn’t working because it was clogged with calcium, which means it was also always low in chlorine, sky high alkalinity and PH and had too much calcium. So I set about fixing all that and had it to the point (after many weeks and lots of salt/money) that all I was battling was the slight alk/pH issue and then my new pool testing kit came in. This kit included the ability to test for cyanuric acid which I wasn’t worried about and, in fact, thought would be low. It was off the charts, literally an unreadable amount, somewhere north of 120. Why? Because I had been shocking the pool regularly with powder chlorine because, like mentioned, my generator wasn’t working and not enough salt in the pool for months. I wasn’t aware that many times pool shock chlorine has cyanuric acid added to it. So, after all that work I am now draining my pool and starting over. At least I know what I am doing now! Sorry for the novel, but I needed to vent!
Hi Ryan, DiChlor shock (Di-Zap) has cyanuric acid added to it, but regular Pool Shock or Super Pool Shock, or non-chlorine shock, does not have it. If unsure, look for the active ingredient. If it starts dichlor-… or is 56% available chlorine, then it is stabilized. You will also see isocyanurate in the chemical name for stabilized chlorine products. Dichlor is used often for fountains or for pools that want to shock during the daylight hours, Dichlor will last longer in the bright sun, but will contribute a few ppm, for every pound added to the pool. Not a problem for most pools/fountains that get a lot of rain, or backwash regularly, or replace water on a schedule. But on others that don’t and use as a daily sanitizer, it can build up in one season, as you have discovered.
Thanks for the reply! I guess it has been maybe 16 months that I have been consistently shocking the pool and also we put a floating chlorine tablet dispenser thing in around a year ago so consistently had 1 to 3 large tablets in that thing too. Is that the only way that cyanuric acid would have been added? Like I said, the results are literally unreadable they were so high, well over 100 ppm. I realize cyanuric acid can be added in it’s normal form but considering I had never even heard of it, I did not add any in that way. All I have added was the chlorine shock and tablets, Morton’s pool salt and muriatic acid in liquid 31% (or around there) form. Anyway, I’ve started fresh and will be very careful about cyanuric acid from now on. I’ve heard to keep it between 15 and 30 ppm?
Ryan, yes it can build up over 16 months, to a too high level, just from using Trichlor stabilized tablets and Dichlor stabilized shock, especially for pools that don’t get a lot of rain, or do a lot of backwashing, or are not closed for winter, with water lowering. Your fill water (from the hose) may also have some stabilizer in it, if it comes from a water treatment plant. Not usually, but in some cases. The normal cya range is 30-50 ppm, but 13-30 ppm is fine as well. PS, I assume that you have retested at least once, to re-verify the cyanuric acid level? Always a good idea, when abnormal or unexpected results are encountered.
I have a 18×48 above ground and the ph is7.2 and alk is 120 but chlorine is 0.5 or sometimes lower, i put alot of liquid chlorine in but its not holding , the stabilizer is 0
Lisa, Let’s ignore the somewhat low pH and somewhat high alkalinity for now. Your pool has about 7500 gallons… when you say you added a lot, how much? For your pool size, every 16 oz of 6% bleach should add 1 ppm chlorine, but it depends on several factors. You could add a gallon to your pool if needed. For cyanuric acid (aka Stabilizer), add 2-3 lbs of stabilizer and that will help you to build chlorine level, if your pool has lots of sun.
Thank you so much for sharing your knowledge and answering questions. First time pool owner here and feeling overwhelmed by ALL of the information that is out there on balancing pool chemicals. We have an above ground Intext 4,440 gallon pool.
Following several attempts to lower alkalinity, we are sitting at 220 alkalinity with a pH of 6.8. In my attempts at balancing these other chemicals, my chlorine has gone back to 0 after shocking. So a couple of questions? What do I worry about first? Do I try to get chlorine back up with another shock treatment and then worry about alkalinity/pH? Or, should I be focused on alkalinity/pH first? I read about the bucket method – wondering – can I use the pH decreaser to lower the alkalinity (while note impacting pH)? Do I need to find a different product? When using the bucket method, do you still follow product guidelines for the amount to use in the bucket? Thank you!!!
Hi Kelly, worry about the chlorine first, add shock, and get it up fast. If your pool is sunny, you may need to add 1-2 lbs of cyanuric acid (Stabilizer), to protect the chlorine from the sun. For the extreme pH/Alk that you have, yes pH decreaser is used to lower pH level and also alkalinity. In your case, of high alk and low pH, you need to add pH up then down, then up then down, it will be a back and forth exercise that can take a week of adjustments. If your fill water is better, you may consider changing some of the water, but it may be close to the same pH/Alk levels? Don’t worry too much about getting exact levels, for an intex pool, which you will likely empty at season’s end. But for swimmer comfort and clear water, do try to get the alkalinity down some, and pH up some.
Hi! We have a 12ft by 30in above ground round pool. I am having such a hard time balancing out our levels. My alkaline will be high and Ph low, then I get our Akaline where it should be and then Ph is still low. My chlorine is still showing 0. I know we have harder water but this is driving me nuts and my kids are losing their patience haha. So we are now at a good Alkaline but low ph. If I add Ph up it makes our alkaline too high. Chlorine never changes. Please help!!
Hi Emily, don’t worry about the pH and alkalinity and calcium – just keep a low pH level. But do worry about a low or zero chlorine level. You should shock the water with 2-3 oz of granular pool shock, then put a chlorine tablet in a chlorine floater, and replace it just before it’s gone, so that you maintain a constant and consistent level of chlorine, of 1-2 ppm, all the time, day/night – that’s most important, along with a pH of 7.0-7.6. Nothing else is so important, for an Intex pool that is.
Hi Davy,
I am a first-time hot tub owner. As per the test strips, the total alkanity reads low(40 PPM), but the PH value reads High (8.4). I have tried adding Alkalinity increaser to push up TA, and then adding PH decreaser to bring down PH. But when I add TA increases, PH goes high (8.4).. When I add PH decreaser, TA drops low. I am confused. What would you recommend as advice?
Hi Mani, that is how it’s done when you are in this predicament, like a see-saw one end goes down the other goes up. What I would do is slam it hard, and lower the pH to around 7.0, and then raise the alkalinity with baking soda. Or, I would first test your fill water to see if it has better chemistry and if so, you could just drain a portion or all of the water, and refill with better water? Regardless, you should drain every 3 months, or whenever you get into predicaments, like this or with cloudy or foamy water, or trouble maintaining sanitizer level, strong smells, strange scum, you get the idea…. 🙂
My pool 24foot above ground,the alkalinity is very high above 240 and the ph is 7.2. How do I lower the alkalinity. Can i use muric acid. Also the chlorine is low. Water is crystal clear.
Anita, let me also ask you if you can test your fill water and see what the alkalinity is, maybe much less than 240 ppm? If very much lower, it could be cheaper and faster to drain 3/4 of the pool and refill. Or you can go the painful route and use the muriatic acid. But you should get the chlorine level up, or the water won’t be clear for long!
We are new pool owners with 15,300 gallon above ground pool. We also have hard water. We filled it and shocked it/ and chlorine tablet in filter. Our water is brown with no scum on bottom or sides. And we can see the floor of the pool. Test strips say our Ph level and Alkaline levels are very high. We added a decreased this morning and no change. I checked the levels again and our chlorine levels are dropping and the ph and alkaline is still high. What do you suggest?
Hi Jennie, keep the chlorine high, and keep adding pH decreaser to get the pH down into the 7.0 – 7.6 range. For the color, brown is nice, but no so much for a pool. A Clarifier may help your filter trap the color. If the color is a metal like iron, you can use a sequestering agent like Stain Away or Super Stain Away, or MetalFree, etc. to tie up the metals in solution. But it’s not a one time treatment, you’d add maintenance doses per bottle label.
My alkalinity is high….took 30 drops to change the color in my water tester…iv added so much acid…my ph is reading zero. My pool water is blue but very cloudy. What do i do?
Samantha, keep filtering, you should run the filter nearly constant. Clean only when the pressure rises or the flow rate noticeably decreases. Don’t worry about the alkalinity for now, just keep a low pH and a high chlorine level, and use a Clarifier and possibly a Filter Cleaner…
Good morning ! I’m having some pool issues ! we have tried a ton of things and nothing is working ! My free chlorine is high at 10( from all of the shock it ans chlorine that has been added ) my ph is low at 6.2 and my total alkalinity is low at 0-40 … the pool has green-like algae at the bottom that is slowly coming up but still there we have vacuumed it/ drained it all but the last foot because it wouldn’t come out … scrubbed it .. and now it’s full of water again and appearing as I stated above .. please HELP! Thank you !
Samantha, just keep at it! Keep filtering, 24/7, the chlorine will come down, but be careful it does not drop to near zero. Use of a Clarifier can be helpful. Add Alkalinity Increaser (Sodium Bicarbonate), to raise alkalinity, which will also raise pH. Get pH above 7.0, but under 7.8. Check your fill water pH/Alkalinity, and if it is much better, you may want to do another partial drain and refill. Fresh water is much more manageable than aged water.
I am a first time pool owner and have hard well water, with lots of minerals. I added Iron Myte while I was filling the water and it is only day 2 of it being filled and it is greenish and murky. When I did the test strip (that’s all I have right now) it said high Alkalinity, good pH, zero free chlorine. I added extra chlorine tabs…not sure what else I should add. Is my water working against the chlorine? Any products that work good with well water?? Thanks!
Hi Casey, filtering will help, it sounds like there may be some solids and minerals and metals in the water. The zero free chlorine is a bit of a problem, so if you can add a granular or liquid chlorine right away, not at a ‘shocking’ level or amount, but enough to get the chlorine level up a bit, like 1/3 lb of pool shock (5.3 oz), per 10,000 gals of pool water. As the water begins to clear, use of a Clarifer can help speed up the final polishing. Afterwards, your filter media may need replacing or cleaning very well.
I am new owning a pool. It’s a 3×12 summer waves. What are the steps I do after adding the water ?
Thanks so much !
Hi Diane, take a look at this post, and then spend some time reading the many comments and replies. https://blog.intheswim.com/what-chemicals-are-needed-for-an-intex-pool/
Hi. We put up a 1100 gallon pool. Using test strips- Chlorine is good. PH says 7.8 But Alk comes back a little high (180). We’ve googled and asked the local pool store with no definitive answer. What can we do for this? Or is it okay this way?
Thank you!
Hi Nicole, not really OK that way, the pool will be much easier to manage if the pH and alkalinity are much lower. However, what can happen is that a high alkalinity makes it hard to lower the pH level. However, even so, I would get a gallon of muriatic acid, or a few lbs of dry acid granular, and add a good dose every couple of days, add 1 cup (8 oz) of acid, liquid or granular. And see where that gets you after 7 or 8 days of treating every 48 hours.
I don’t trust my eyes when it comes to test strips so every week when I take my water sample to the pool store alkalinity is always low (60-71)and ph is on the high end (7.6-7.8).
Seems like I’m constantly adding 3-4 lbs of alkalinity up each visit then a little acid to knock the ph down.
What could be causing this? It doesn’t seem normal. Ph of my makeup water maybe?
Jay, if your water is clean/clear and you don’t have clarity or sanitation issues, you could be fine to operate at a lower alkalinity and higher pH level. But above 7.8 or below 60 ppm alk, and I would adjust. It is perfectly normal for pools to fluctuate with these two, depends on the pH of other chemicals used, debris and rain received. Even wind blown dust can affect the water, as well as other water balance parameters and water temperature. You might find it interesting to conduct a Langelier Index analysis on your pool, to see how a pool with slightly low alk, and slightly high pH, can still be in balance. https://www.pentair.com/en/knowledge-base/pool-spa-equipment/pentair-pool-calculators/saturation-index-calculator.html
My TA is high. my pool supplier is having me add pH up then acid to bring the TA down. I have been doing this and have noticed the “seasaw” effect this is having so found your site. On the bucket method can I use liquid acid and carefully lower the bucket? or need dry acid? my TA is now running 250. pH at 8.2 and I was going to “walk” the acid. please advise. very frustrating. ready to dump water but that be about 12,000 gals.
Steve, yes you can use liquid acid and carefully fill the bucket with water and 1 gal acid, and set on a step. The dry acid might be more effective, in this method, not sure. Yu can also try some aeration as a natural way to raise pH, if only by 1 or 2 clicks. Using a floating fountain, or or other sprays, and lots of cannonballs!
I’m using the bucket method to lower TA without affecting my already low pH level.
I filled a bucket with water mixed in pH minus, then set the bucket on the bottom step and set a timer for three hours.
How do I go about retreiving my bucket when time is up? Do I use a pile to lift it by the handle? Do I dump the water I to the pool? Do I just leave the bucket and turn the pump back in?
I guess you could just walk in / reach in to the pool and grab the bucket handle, and then dump it in the pool. The idea is that all of the chemical will have slowly come out of the bucket, and for those readers wondering what this is all about, this ‘bucket method’ can be used to affect alkalinity more than pH. The saying goes, to lower alkalinity more ‘pool’ your acid, to lower pH more ‘walk’ your acid, or just pour it in as you walk around the pool. Pooling the acid is supposed to create a greater exchange of hydrocarbons, affecting the bicarbonates and carbonates more, or something like that..>>! By putting the dry acid in a bucket and setting it on the step, this is a way to ‘pool’ the acid.
Opened 15,000 gal pool and added water-we have natural high alkaline in our city water so each year usually need to add 1 1/2 gal of muriatic acid to lower and then use sodium carbonate to raise Ph. In the past this has always worked but this year after lowering the alkalinity 100ppm started to slowly raise Ph so Ph is perfect but the alkalinity jumped to 180ppm. Repeated the process and a back to perfect Ph but high alkalinity!?!? Suggestions?
Hi John, keep at it? You may also be able to use Aeration of the water, with a fountain or spray jet, or pointing eyeballs to the surface, (or cannonballs!), to slightly raise the pH naturally.
Questions regarding the Bucket Trick:
1. My plastic bucket will float and then turn upside down. Is this wanted or do I weight the bucket with say a brick or something?
2. The trick says to use clean water. Does that mean clean pool water or does that mean tap water?
Thanks for your help!
Hi Don, fill the bucket with more water or use a larger (5-gal) bucket, that should hold it down (or you can use a brick, sure). And you can just use pool water, yes.
Davy,
Thanks for your prompt reply.
So I take it from your answer that the bucket is not fully underwater? If that is the case, I assume I would just spread the bucket contents around the pool afterwards.
I do have a 5 gallon similar to the one in your picture.
Thanks again
See this post: https://blog.intheswim.com/pool-leaks-how-to-find-them-and-fix-them/
Hi. Thank you for sharing your knowledge. I have an 11,500 Gallon pool and cannot get it balanced at all. this has never happened in all my years of owning it. We closed in winter with a perfectly balanced pool, used the appropriate winterizing kit, and opened without any debris or leaf matter or issue. The ph was high and alkalinity was borderline ok/high, so I added the correct amount of ph down. Now my ph is below 6.2 and the alkalinity is the same (So I lowered it too much I guess? Even though it was only 1lb). I added ph up and am getting no results after several hours, but am reluctant to add more. We bought new test strips which match the old ones’ reading. No matter how much I shock, I have only been getting zero readings on my chlorine (we did add cya in a sweat sock 3 days ago, which has dissolved but still is reading zero As well). The pool store recommended 1/4 of an ounce of Muriatic acid, but my ph is so low already, I’m afraid to add it in. We took apart, cleaned, and am running the filter 24/7. I have vacuumed daily and scrubbed the sides and bottom. I’m feeling pretty hopeless at this point. We’re going on a week and cannot get the pool safe to swim in. Thanks for your expertise.
Add more pH increaser – the high alkalinity is being resistant to change, so add a double dose of pH up. For the fee chlorine, try a triple dose of shock, or 4 lbs, all at once, and see if that will break-thru the invisible consumer of your chlorine. Go ahead and get in the pool, it won’t hurt you!
Hi I just open my pool shocked it put everything in it everything is fine but the free clorine what do I need to put in that pool to raise up the free clorine because I’m trying everything !!! Please help
Hi Crystal, you would add chlorine shock to raise the free chlorine level fast. IF you are adding chlorine, but not getting any reading, and you are sure your test strip or reagents are not expired, then you may need a triple dose of shock, maybe more, to break apart whatever is consuming the free chlorine.
Hi there. I have a 200 gallon kiddie pool that does not have a filtration system. I also live in California and need to keep dumping the pool to a bare minimum. So, the water straight from the hose is 0 chlorine, Alk is in good range, pH is also in good range, CyA is also in good range.
So my question is, do I add chlorine, and then what about if it changes alkalinity or pH? I’m planning to use 1″chlorine tablets and breaking up arm and hammer clear balance tablets in a floater. Will that be enough to maintain the pool water?
Hi Teresa, see this recent post on managing inflatable pools: https://blog.intheswim.com/how-to-maintain-an-inflatable-kiddie-pool/
24,000 gallon in ground pool.. ph is 7.8, TA is 20 ppm. How do I fix this
Hi, First add 20 lbs of alkalinity increaser, all at once. After 8-hours, use 4 lbs of pH decreaser, or dry acid. Then recheck levels. You may need to repeat, a few more times, until you get it right, with adjustments to quantity added.
I have 6000 gal. Pool. My ph is below 6.8 (my tester does not test below this, hydrotools by swimline) this tester recommends adjusting ph prior to alkalinity, everything i read is oppisite this thinking. When i use this tester for alkalinity test i get pool water, add drop of de-clorination, then add alkalinity test solution and water should turn blue, my water never turns blue. Not sure what to do
Sounds like your alkalinity is high and pH is low. High alkalinity is making your pH very resistant to change. Normally, it is ‘Alkalinity first’. Add 2 lbs of pH increaser (Soda Ash), and see if that will get your pH to 7.2, which would be fine. And over time, work on lowering the alkalinity, with large doses of pH decreaser, and then raising pH with soda ash and aeration. Getting some oxygen into the water will naturally increase pH a small amount.
Davy,
I’ve surrendered on the cloudy water this spring. 20K inground vinyl. Remains cloudy at a constant level. Can just see shallow end floor. Ph steady at 7.6. Alkalinity at 150. Checked filter for leaks. Checked manifold fit. Checked spider gasket. Vacuumed pool and shocked 3x @ 2-3 lbs. each. Used clarifier 2x. CC is 0 with FC of 2-3. Used both de and perlite. Only environmental factors are pollen and bird poop.
Hi Dennis, okay you have tried many good things I can see… let’s also consider that the alkalinity may be the culprit, at 150 ppm. Get 2 gallons of muriatic acid, and start hitting that alkalinity. This will pummel your pH, so you’ll need some pH increaser on hand. second, consider that maybe calcium scale is the culprit, and if you try a Stain & Scale chemical like our Stain Away, or the numerous other sequestering agents, this will tie up precipitated calcium, or dissolve it back into solution. Third, it could be a TDS problem, with very high total dissolved solids, which can cause consistently cloudy water. I’m assuming the filter runs for a good run each day, at least 12 hours… what happens if you go 24/7 for a week?
Hello Davy, I just had my indoor pool replastered, 16,100 gallon pool, end of March 2020. I been fighting high PH levels ever since. The problem is my ALK keeps dropping further down the scale as I try to lower PH to acceptable level. I wrote the pool company that did the work on my pool and asked if they sold anything that will lower PH but not ALK. They sold me a 6 lb bucket of PH Minus. This still lowered my ALK further and yesterday I did another check and now have a PH of 8.2 with ALK of 62. I mixed a half gallon of acid in a bucket of water and dumped it around the pool in four or five different spots to try and lower the PH, pool pump running. I know this is now going to drive the ALK further down. So to help with that, I live in the country and have a well. I added water knowing the incoming water is high in ALK. I haven’t checked in since. The pool water looks amazing and is great to swim in. I also use no Chlorine. I have an ION unit on the pool which is right where it needs to be when testing. Any suggestions or approaches would be greatly appreciated 🙂 Thanks
Hi Dave, new plaster coats will produce high pH for the first year. What you have to do is Raise the alkalinity, using Alkalinity Increaser, while also adding acid to lower pH levels. Adding water from the well will help also. Be sure to use a Stain & Scale chemical to control minerals and metals from the well water.
So I will need to add both acid and Alkalinity Increaser for the next year and it should level out then? Both at the same time is ok? I started mixing the acid with pool water in a bucket and dumping it in different spots, pump running. I was told pouring straight acid in the deep end with no pump running will drop ALK faster which is not what I want.
What do you recommend for a Stain & Scale? Being an indoor pool in a cold weather state, Wisconsin, we do keep a solar cover on it when not in use. Holds the humidity down. We notice a white like powder that forms on top of the cover. It seems to disolve when get in the water again but looks kind of gross. We were not sure if this was coming because the plaster was bad. We haven’t noticed it since the plaster. But it only been a little over a month and usually takes longer to form. Would this help with that? I am thinking it was calcium. Being well water our hardness is around 300 to 400 ppm. But I was told that should be ok. I did just pipe in soft water fill also. So I can use either soft or hard water now from the well. Thoughts?
Yes, you adjust pH, then alk, then pH, then alk, until you get it right. But you don’t add at the same time, give it a few hours between doses. That’s true, ‘walk’ your acid to lower pH more, ‘pool’ your acid to affect alkalinity more, but the effect may be minimal. The white powder may just be water evaporating from the top of the cover and leaving behind the calcium. I don’t think that a stain & scale will treat that, or stop that from happening. But, I’ve been wrong before! You could try our own In The Swim Stain & Scale, so you’re not spending too much on the experiment. Let us know how it works.
Good morning, I enjoyed reading the article you published about PH and Alkalinity problems. We just got a new pool,mineral packet, thriclor tablets, marcite, approx 25K gals. As expected, PH keeps going up because of the new plaster. Total alkalinity is way low, 57ppm as measured in a pool store (test kit recommends 120-140ppm as the ideal range for Thriclor tablets, and 80-100 for liquid chlorine…thoughts on that?).
Anyway: over the last 2 day, I ended up (progressively) pouring an entire 30lbs of alkalinity increaser. As expected, PH got a little higher, but to my surprise, I have been retesting Total Alkalinity and nothing has changed! ( I am referring to the test I have at home, I have not take the water to the pool store yet). What I find a little concerning is the fact that the pool store recommended only using 17 lbs of alkalinity increaser to drive alkalinity up to their recommended range (80-120ppm)…I used double that and nothing changed…as I read the comments above, they make me wonder: if the new plaster keeps driving PH up but I add acid to drive it down, sounds like I also drive alkalinity down. So you recommend waiting a few hrs between adjusting PH and alkalinity…but basically in my case, add acid to lower the PH and add alkalinity increaser to fight the imbalance. Do you have a recommended dose to alkalinity increaser? the testing kit has a chart, but only works when you are pretty close to the range….my pool’s alkalinity is way off the charts…
The reason for the difference in alk levels for different types of chlorine is related to the pH level of the chlorine. Trichlor tablets are low in pH while liquid chlorine is high. So, using tablets over time will tend to lower both pH and alkalinity. But 120-140 ppm may be a little high, IMHO. I would shoot for 100-120 ppm. Higher alkalinity levels will hold your pH level more stable. 30 lbs shoulda had some effect, maybe the store test will reveal?
I use the Power Ionizer. PH is 8.0 and TA is 125. Hardness is 92ppm. Should I even mess with the PH and TA? Everything is crystal clear with no visible issues. Also, should I increase the calcium hardness?
Yes, the high pH will make chlorine less potent, and algae thrives in pH above 8.0. I like 7.2 – 7.6. TA is fine at 125. Hardness at 92? That will make the water more susceptible to foaming, and it is hard on the pool liner, over many years of such soft water, and it can harm soft and shiny surfaces like vinyl or rubber. Raise calcium to 150 ppm minimum, and lower the pH to 7.6 maximum. Have a great summer!
I have a 30k gallon saltwater gunite pool with a salt cell. I had the water levels pretty good a week ago. Alk. 90; Ca hardness 225; salt 3400; and pH 8.0. I added a gallon of Muriatic Acid and lowered the pH to 7.6 but also lowered the Alkalinity to 80 ppm. But now the pH keeps going back up to 8.0-8.2 after a week or so, but the TA is holding at 80ppm. So if I keep adding acid it will keep lowering my TA which is at the low end now anyway. Any suggestions??
You’ll need to add Alkalinity Increaser in addition to your pH decreaser. The salt system tends to raise pH levels in pools.
Hi Sharon, 7.3 is good for pH. alkalinity is good from 80-150 ppm. chlorine must be 1.0-2.0 ppm all the time, so – what about stabilizer level, cyanuric acid? a level of 25-50 ppm is good to help in hot Texas sun. Not so important in shady, northern pools, but that may be your trouble in maintaining a chlorine level. Converserly, on the opposite end of the spectrum, if your stabilizer level is too high, over 100 ppm, that can also cause a problem with testing a good chlorine level. Another cause is something in the water that is literally consuming your chlorine, and it often takes a heavy shocking to bust thru and restore order. Circulation is also important, with a pool so deep as yours, Try to aim one of the returns to sweep thru the deep end bowl, and run the cleaner a bit more, or brush weekly, if you think the deep areas need more circulation. Baking soda will raise pH but not as much as Soda Ash, baking soda raises Alkalinity more, and pH less, and it’s the reverse for Soda Ash. Your pH of 7.3 is perfect. 🙂
Hi, My alkaline and ph levels are high. Chorine correct. If I had PH minus it reduces alkaline and ph but also reduces chorine. I then add more chorine and alkaline and ph increase. Seem to be going round in circles. Do you know why this is happening?
Hi Guy – the chlorine is not really related, or does not affect your ph/alkalinity. but ph and alk both affect each other greatly, Best ranges are pH 7.2-7.6, alkalinity 80-150 ppm. If your pH and alk are both high, then add until you get your pH to 7.0 or 6.8, and then add a pH increaser (Soda Ash), which will also raise alkalinity a little bit more. Then add acid again, which will depress both pH and alk, and continue in such a fashion as to see-saw to a better place. It’s ok if your alkalinity is a little high, but I like to see pH 7.2 – 7.6. You can also use aeration to raise pH naturally (slightly).
Question. I have an indoor pool with a PH level at 7.5 but alkalinity of 220. I can’t figure out a way to lower my alkalinity to a usable level while not destroying my ph levels. What’s the best way to fix this issue
Hi Bryan, is it an issue? Is the water going cloudy frequently? 220 is high, but in some cases, it may pose no problem. If so, then just keep adding acid, dry or liquid, to lower to 150 ppm or so, it will just require several treatments, maybe 3-5 x
The high alkalinity actually burns my eyes after an hour out of the pool. Plus gives some skin irritation. I think my only option is to slowly lower the alkalinity with ph minus while trying to gain some PH back with aeration. Do you know of any other options
Hi, the joker in me wants to suggest Aqua Seal goggles 😉 but seriously folks, howis your chloramine level? That’s normally the cause for eye irritation, although I suppose pH and alkalinity is also a cause, yes. The method you describe is good, I prefer to try and swing for the fence, or hit it heavy the first few times, as needed, and then use smaller doses to fine tune it. Test 2 or 3 times per day, adjust if needed, and in 2 or 3 days, maybe more, you should see some marked improvement.
Hello. My pool water has total alkalinity of 150 ppm and pH of 7.6, and I am trying to figure out what I need to do to reduce the amount of calcium scale that builds up along the waterline without affecting the pH too much. We had the scale bead blasted off and then a couple of weeks later there was a faint line of calcium scale appearing again. Any suggestions?
One thought is to seal the tile, we have a line of products called LayorCare, and Layorcare Cal Block is such a product, to seal the surface so the calcium won’t stick. Now that doesn’t mean it won’t stick in other parts of the pool, just not at the waterline. Are you using a sequestering agent regularly, a Stain & Scale liquid? I would recommend that too, Natural Chemistry Scale Free https://www.intheswim.com/p/scale-free is a good one for this particular issue. These chemicals deplete, so they need to be redosed every few weeks, but help to keep minerals in solution, where they cannot deposit. Third is to get softer water, one way is to have the water purified by a truck mounted reverse osmosis filter (available in certain parts of the US), or you can drain and refill with some amount of water that is softer, wither from your hose, or delivered by truck, or if you have a home water softening system, fill the pool with treated water
Hi there! My pool water ph is about 7.4, but TA is low, about 60ppm. How can I raise the TA to the correct level without raising the PH too
much please?I have tried adding Soda Ash before to raise the TA, but everytime I do that the ph goes way too high.
Thanks!
That is one of the ‘funny’ things about pool chemistry, is this, so you just have to do it in stages, raising, lowering, raising, lowering, and so on, until you get it right. allow 1/2 day between adjustments, and you’ll get there, eventually
Alkalinity remaining relatively the same after 3 applications of ~4lbs of Alkalinity Increaser. I used LaMotte test strips, which showed lower end of acceptable range, but LaMotte Digital and Leslie Pool store show lower levels (~30 LaMotte; ~50 Leslie). What could cause the alkalinity levels to remain the same?
Thanks for responding. I finally figured it out. It was a bad Alkalinity reagent from LaMotte 2056 ColorQ Pro 7. The 7038 reagent was replaced by 7039…thankful they sent it, but after I must have dumped over 15 lbs of Alkalinity increaser. I was almost thinking the Alkalinity Increaser was bad. I got a reading of 155 today, but I also added another ~5lbs of Alkalinity increaser in the morning, so well see how it reads tomorrow. I am still waiting for In the Swim to finally send me my pH Reducer, but if it’s real bad I’ll get some Muriatic Acid. I am hoping after a backwash and refilling the pool with water from hose will also help. Anyway, now I can start adjusting calcium hardness.
So, it’s been a week and my Alkalinity is still high at about 160, but all the Dry Acid I added lowered the pH to 7.1. Not sure if I should keep lowering Alkalinity, and then raise pH. Current readings: FCL 3.36, TCL 4.48, pH 7.1, AK 160, CH 160, CYA 86.
It would be OK to push the pH into the acidic range – down to 6.0 briefly, so you could add more pH down, then use pH increaser to raise the pH back up.
Thanks for the advice! I have gone through a lot of the chemicals I ordered through In the Swim (Alkalinity Increaser, Dry Acid, and Calcium Hardness Increaser) to get my pool close to swimming levels. I know there are safe pool operating levels and there are safe swimming levels. Can you tell or point me to a chart type listing the safe swimming ranges (though they may not be ideal for pool operation)?
My last reading was about:
FCL 4.5
TCL 5
pH 7.2
AK 140
CH 170
CYA 95
I added 8lbs more calcium hardness increaser and I am replacing about 3-4 inches of water in my 15,000 gallon pool.
The .5 difference between free and total chlorine would indicate a need for shocking. I would replace more than 3-4 inches, but replace half of the water if possible, to get the CYA reading down to 50 ppm. pH/Alk/calcium all look fine.
I’m trying to use the water in my kiddie pool for longer than a day (California = Drought). I put some chlorine in and the chlorine level is good but the alkalinity is very high. This pool does not have a filter. How can I reduce the alkalinity and the pH without the pool having a filtration system?
Hi Jill, add a pH decreaser to the pool, either muriatic acid, or dry acid (sodium bisulfate). We sell the dry acid, called pH decreaser, and it will lower both alkalinity and pH levels at the same time. You can use poolcalculator.com to compute the amount of acid needed for your size pool, and add it directly to the water, and then test pH again a few hours later. High pH makes chlorine very sluggish and ineffective, and may not be controlling bacteria they way it should be, so good question. See also my new post on how to maintain an inflatable pool: https://blog.intheswim.com/how-to-maintain-an-inflatable-kiddie-pool/
The pool people said I needed 25 lbs alkalinity, my ph was fine, just add the alkalinity then shock with 8-10 bags of shock (36,000 in ground pool, just opened). Can I use 25 lbs baking soda instead of alkalinity?
Hi Tammy, yes you can, many times Alkalinity Increaser is sodium sesquicarbonate, but other times people using sodium bicarbonate, or baking soda. Yes, you can use regular Arm & Hammer… be sure to check pH level afterwards, as it will also raise pH, and for shocking, a lower pH is best, as chlorine is more potent. You may wish to shock before adding the baking soda, for greater efficacy.
How fast could you raise PH if you ran a pool closing blower through the Main Drain line?
Hi Dan, not sure as I’ve never done any experiments with aeration to raise pH. I don’t believe that it will raise it very much, maybe 2 clicks, and probably not very fast, might take 12-hours or more? Let us know, if you decide to do it! I’d be interested to know…
Hi Davy, my TA on my hot tub always stays between 20/40 and my PH stays around the 7.4.
When I raise my TA the ph goes through the roof, I have tried setting TA first then loading the PH but it throws it all off? Can you help please?
Your help is appreciated
Maurice
Hello, with a hot tub, you can run the alkalinity lower and be OK, if you can maintain 30-40 ppm of alkalinity, 7.4 pH and run the calcium a little high, 250-400 ppm, your water will be balanced. Don’t worry about getting alkalinity to 80 ppm, as long as your pH level is steady and mid-range.
Hi Davy, I have a pool/spa with Pentair’s Intellichem (chlorine/Muriatic Acid) & UV Sanitation. I also have a SCG, but haven’t added salt since refilling the pool in Jan. My PH stays right at 7.4, but consumes a little over 2gals of acid a week. My Alkalinity will start at say 100 and drop to 60 over a week or two. The two seem to be in constant conflict. I refilled the pool as the CYA was at 60 and my system wants under 30, now at 5, but my PH & Alkalinity were stable. I raise the alkalinity with baking soda 14lbs which will bring it back to 100/110. Any thoughts?
Thank you!
Hi Chad, the SCG (salt system) is likely the primary cause of the high pH. If you decided to switch to tablets, you would not need so much acid, which is what lowers the Alkalinity. adding the Alkalinity Increaser (14 lbs) will also raise the pH level. It’s a bit of back and forth between the close cousins pH/Alk, until an equilibrium can be found. With the UV system running, you can use less chlorine than a pool without the supplemental sanitizer.
Do these tricks work the same for hot tubs? I’m struggling to find the balance. Alkalinity is perfect in the spa but PH is high…
Hi Stephanie, yes the same applies for hot tubs as for pools. Spas are much smaller of course and can fall out of balance more easily. Rising pH is common after using the spa, or if certain high pH chemicals are used, like liquid chlorine. Some spas need near daily adjustments to keep the pH in-line.
Hi
I have a combined spa/small pool (900l and 7000l) and having trouble keeping chemicals balanced.
pH always high and Alk low (8.2/50).
How can I get one right without affecting the other?
Thanks
HI Scott, it’s a common and unfortunate problem, to have high pH and low alkalinity. The only way to correct and balance the two is by adjusting one, then the other, repeatedly, until you get them down into proper range. Lower the pH using sodium bisulfate or muriatic acid, and then raise the alkalinity using sodium bicarbonate. It may take 5 or 6 adjustments, but you’ll get there eventually. And once the alkalinity reaches 90-100 ppm, it will hold the pH steady, in the 7.2-7.4 range. Using chlorine tablets (instead of liquid chlorine) can help, as liquid has a very high pH level. Good luck, Scotty!
We have a 1 year old 16K gallon saltwater gunite pool and enjoy balancing the water myself. I have been able to keep PH in control with about 48 oz of Muriatic acid each week. Alkality seems to be on the low side all the time even though I added 5# baking soda one day and another 5# the next. I got it up to about 80 I guess but the next week it’s down again. (Used to have a 25K gallon pool previously and don’t remember hardly ever having to add baking soda) Do I just need to get Alkalinity much higher and then everything will settle in better? Thank you!
Hi Renee, the issue occurs because the salt system creates sodium hydroxide as a byproduct, which is very high pH, and will raise your pH over a week’s time. Adding the muriatic acid pummels your alkalinity level, requiring baking soda…. Not fun, try to add acid in smaller doses, avoid heavy dosing all at once, but spread out the dose in two half-doses, at night, and the next morning for example. Add more baking soda, in larger doses, all at once. 10 lbs should raise 40 ppm in a pool your size.
I thought aeration increases the pH, not lowers? I have the same problem of chemistry as this person. Alkalinity of 80, and my pH shoots back up to 8.2-8.4 every week. I thought my pH was staying high because of my spa fountain that runs continuously with the return when my pump is running. Can you clarify the aeration lowering pH? Also my pool is about 7 months old.
Hi John, you are correct, aerating pool water will slowly raise pH, not by much, but some. You could shut off the fountain for a few weeks and see what happens, but I would suspect something else contributing, like a salt system, or liquid chlorine, or new pool plaster, or some other cause.
Hi Davy,
I have an above-ground pool that I’m struggling with. The pH is reading about 7.0 and yesterday, the alkalinity was 30ppm so I added sodium bicarbonate to adjust. I guess I way overdid it, because today the TA is around 240. The pH is still hovering around 7. What should I do? I was thinking acid to bring down the TA and pH increaser once TA is leveled out, but now I’m afraid to overcorrect again.
Hi Lan, if easy to do, I would lower the water level by half and refill the pool to correct the alkalinity problem. Otherwise, you can use acid to lower the alkalinity, but then you have to raise the pH, then lower the alkalinity, in a see-saw process, until restored. It can take 3 or 4 treatments. First raise the pH from 7.0 now by adding pH increaser, to raise near 8.0, then hit the alkalinity. A good test kit can be helpful, test strips can be inaccurate by 20-30%.
My pool got really cloudy after a heavy day of swimming. I shocked it but it kept getting worse, was almost murky, couldn’t see bottom. Took a sample to pool store. They said the ph and alkalinity had bottomed out. They said to shock it again and over a 3 days I put in 15lbs of alkalinity enhancer.24hrs after last treatment it tested really high.put in ph
Minus I tested it again, levels still seamed high but the pool place said they were perfect said to shock it again and put in clairifier. That was almost 24hrs ago. My pool is still cloudy, I still can’t see the bottom. What do I do?
Hi Emily, that is most likely related to low chlorine levels and poor filtration. Your filter may be struggling, or it may not be run long enough per day. Some small filters need to run 24/7, esp. during hot weather and days of heavy use. Chlorine level should be a consistent and constant 3-5 ppm when trying to clear cloudy water, that’s really the most important chemical measurement, in such cases…
My ph is 7.2 but my alkalinity is 180…..what to do ? The bucket trick ?
Hi Karen, well just keep at it! If you have ever thought of buying a floating pool fountain, now may be a good time. Using a fountain will aerate the water to raise pH naturally. It’s not super effective, but will help the process. Just keep dropping the pH with pH reducer to lower alkalinity, and then raise it with aeration or pH increaser. If you can get it down below 140 ppm, that may be good enough.
Our pool is crystal at the moment coming out of winter/spring. I have kept up the vacuuming and chlorine, unlike previous years where I let it go a bit. We have a Nature 2 and I need to renew the cartridge before the weather bites.
However, I don’t understand the advice I get from the local pool shop. Every time I go in (weekly/fortnightly), it’s ‘you need 4kgs of Alkaline.’ Every time. Nothing about acid, whereas the previous owners never recommended huge doses of Alkaline – ever – there was the occasional recommendation to chuck in 300 mls, or so, of acid.
Nothing from these guys on acid – I have followed their advice for the last two years, but wonder if I am being ripped off with ‘you need 4kgs of Alkaline’ every time I go for a water test.
Hi Peter, do they give you a report showing the test results of your water? That is a standard practice in most stores, in the US anyway. The recommended level for Total Alkalinity is 80-120 ppm in most cases, so as long as your water has at least 80-90 ppm, there is not usually a need to add Alkalinity increaser. Free water testing from stores is of course a sales tool, so the best way to avoid buyer’s remorse is to validate with your own water tests – or get the same test kit the pool stores use, Taylor K-2005 and test yourself, it’s not very hard to do…
Quick question regarding winter algeacide. I didn’t know it existed until after I have closed our above ground pool. I’ve drained the pool down below jets, drained the filter and lines, and removed lines for the winter. I’d like to add the winter algeacide but without the pump hooked up, can I pour some in the pool and manually mix it in by brooming around the pool?
Hi Rick, sure no problem. Our winter algaecide will disperse easily, just open up the pool cover along one side, or at several spots around the pool, and pour it in. No need to brush after.